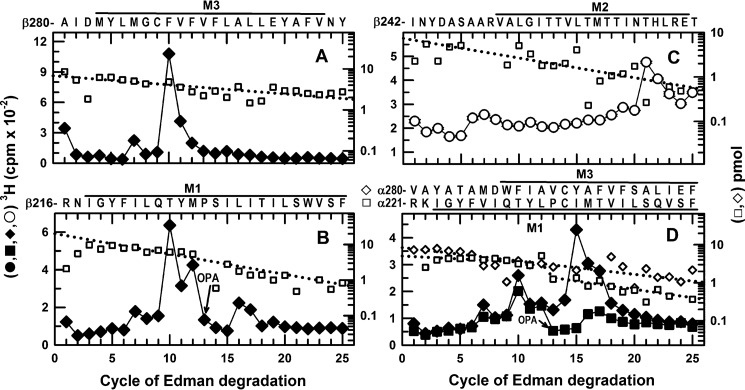
FIGURE 9.
In α1β3 GABAARs, S-[3H]mTFD-MPPB photolabels β3Thr-262 (βM2), β3Met-286/β3Phe-289 (βM3), and α1Met-236 (αM1) at the β+-α− interface and β3Met-227/β3Leu-231 (βM1) and α1Tyr-294 (αM3) at the α+-β− interface. 3H cpm (♦,■, ○) and PTH-derivatives (□,♢) released during N-terminal sequencing of fragments beginning before βM3 (A), βM1 (B), βM2 (C), and αM1 and αM3 (D) from α1β3 GABAARs photolabeled with 2.7 μm S-[3H]mTFD-MPPB in the absence (♦, ■) or presence of GABA (○, 300 μm) or bicuculline (data not shown, see Table 1). A, when rpHPLC fractions 26–27 were sequenced from an EndoLys-C digest of the 61-kDa gel band, the fragment beginning at β3Ala-280 was present at 6.8 pmol, and the peaks of 3H release in cycles 7 and 10 indicated photolabeling of β3Met-286 and β3Phe-289. B, when rpHPLC fractions 28 and 29 were sequenced with OPA treatment at cycle 13, corresponding to β3Pro-228 in βM1, the primary sequence began at β3Arg-216 (I0 = 20 pmol) and a secondary sequence began at β3Ala-280, present at 2 pmol before OPA and undetectable after treatment. The peaks of 3H release in cycles 12 and 16 indicated photolabeling of β3Met-227 and β3Leu-231 in βM1. The peaks of release in cycles 7 and 10 resulted from the photolabeling of β3Met-286 and β3Met-289 in the secondary sequence present before OPA treatment in cycle 13. C, to identify photolabeling in βM2, aliquots from the 59-kDa gel bands were sequenced after treatment of the sequencing filter with BNPS-skatole to cleave at the C termini of tryptophans. The sequence beginning at β3Ile-242 was present (I0 = 7pmol), along with fragments beginning at the β3 subunit N terminus, β3Arg-68, β3Val-93, β3Arg-169, and β3Ser-427 at 4–10 pmol each. The peak of 3H release in cycle 21 indicated photolabeling of β3Thr-262 (βM2–12′). D, 2 aliquots of rpHPLC fractions 26–29 from an EndoLys-C digest of the 56-kDa subunit gel band were sequenced with (■) or without (♦) OPA treatment in cycle 13 (at α1Pro-233). For the untreated sample, the fragments beginning at α1Arg-221 (data not shown) and α1Val-280 (♢) were present at 11 and 8 pmol, respectively. For the OPA-treated sample, α1Arg-221 (□) and α1Val-280 (data not shown) were initially present at 5 pmol. After OPA treatment, sequencing of the α1Arg-221 fragment continued, although the α1Val-280 fragment was reduced by >90%. The peak of 3H release in cycle 15, not seen after treatment with OPA, indicated photolabeling of α1Tyr-294 in αM3. After treatment with OPA, the small peak of 3H release in cycle 16 indicated photolabeling of α1Met-236 in αM1. Efficiencies of residue photolabeling in the absence or presence of GABA or bicuculline are included in Table 1.