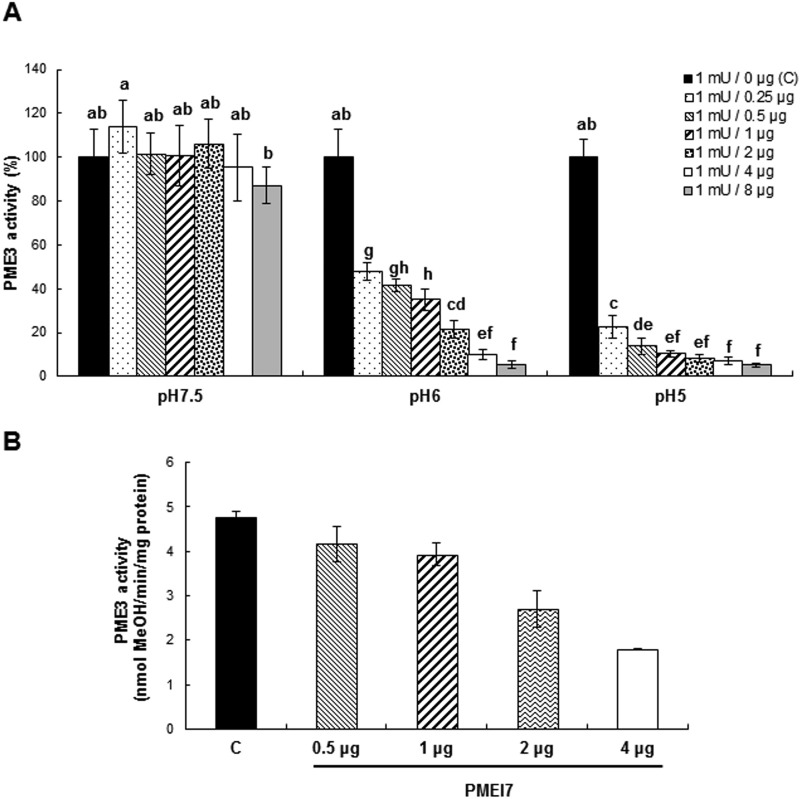
FIGURE 5.
Inhibition of PME3-His6 activity by PMEI7-His6 due to pH-dependent complex formation.
A, quantification, using a gel diffusion assay and a substrate of DM 90%, of the pH dependence of the inhibitory capacity of PMEI7-His6 on PME3-His6 enzymatic activity. 1 milliunit of PME3-His6 activity (corresponding to 2 μg of protein) was used with either PMEI7-His6 conservation solution (■) or a PME3-His6 activity (milliunits)/PMEI7-His6 (μg) ratio of 1:0.25 (lightly dotted bars), 1:0.5 ( ), 1:1 (
), 1:1 ( ), 1:2 (heavily dotted bars), 1:4 (□), and 1:8 (gray bars). Results are means ± S.D. (error bars) of six replicates. The different letters indicate data sets significantly different according to Tukey's range test, preceded by a one-way ANOVA having p < 0.001. At the most acidic pH, the maximum inhibition of PME3-His6 activity is reached for a ratio of ±60 pmol of PME3-His6/60 pmol of PMEI7-His6. B, inhibition of PME3-His6 activity by PMEI7-His6 on the HG96B82 substrate. PME3-His6 and PMEI7-His6 were preincubated for 30 min at 30 °C at pH 6.0. HG96B82 was added to the mixture and incubated for 30 min at 30 °C. The reaction was stopped at 90 °C for 10 min. PME activity was determined using a procedure adapted from Ref. 43. 1 μg of PME3-His6 was used with either PMEI7-His6 conservation solution (■) or a PME3-His6 activity/PMEI7-His6 (μg) ratio of 1:0.5 (
), 1:2 (heavily dotted bars), 1:4 (□), and 1:8 (gray bars). Results are means ± S.D. (error bars) of six replicates. The different letters indicate data sets significantly different according to Tukey's range test, preceded by a one-way ANOVA having p < 0.001. At the most acidic pH, the maximum inhibition of PME3-His6 activity is reached for a ratio of ±60 pmol of PME3-His6/60 pmol of PMEI7-His6. B, inhibition of PME3-His6 activity by PMEI7-His6 on the HG96B82 substrate. PME3-His6 and PMEI7-His6 were preincubated for 30 min at 30 °C at pH 6.0. HG96B82 was added to the mixture and incubated for 30 min at 30 °C. The reaction was stopped at 90 °C for 10 min. PME activity was determined using a procedure adapted from Ref. 43. 1 μg of PME3-His6 was used with either PMEI7-His6 conservation solution (■) or a PME3-His6 activity/PMEI7-His6 (μg) ratio of 1:0.5 ( ), 1:1 (▨), 1:2 (heavily dotted box), or 1:4 (□). Results are the means ± S.D. of two replicates.
), 1:1 (▨), 1:2 (heavily dotted box), or 1:4 (□). Results are the means ± S.D. of two replicates.