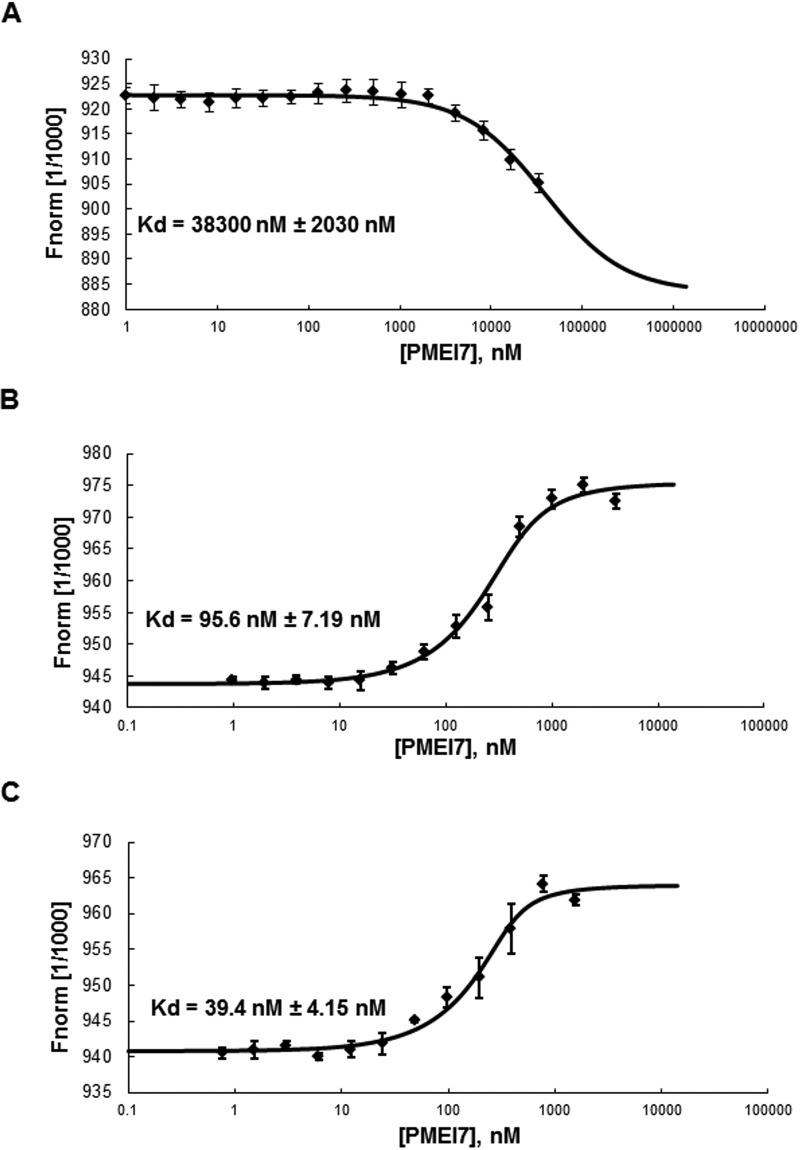
FIGURE 6.
Molecular interaction between PME3-His6 and PMEI7-His6 according to pH. The interaction between PME3-His6 and PMEI7-His6 was determined by MST. For the MST binding assay at various pH, PME3-His6 was labeled by a fluorescence blue dye, covalently attached to the protein. The concentration of the blue dye-labeled PME3-His6 was kept constant (333 nm), whereas the different concentrations of the non-labeled PMEI7-His6 between 33,000 and 1 nm, between 4000 and 0.98 nm, and between 1562 and 0.76 nm were used for pH 7.5 (A), pH 6 (B), and pH 5 (C), respectively. Mixtures of blue dye-labeled PME3-His6 and titrated non-labeled PMEI7-His6 were incubated for 15 min at room temperature and loaded into standard capillaries for MST experiments with 90% LED power and 40% MST power as thermophoresis conditions. The fit curve and the resulting dissociation constant (Kd) values were calculated by averaging replicates assimilated using NT analysis software. Kd values represent the mean ± S.D. (error bars) from 6–8 replicates, depending on pH. Concentrations on the x axis are plotted in nm. Kd of 38,000 ± 2030 nm, 95.6 ± 7.19 nm, and 39.4 ± 4.15 nm, respectively, were shown for pH 7.5, 6, and 5.