FIGURE 3.
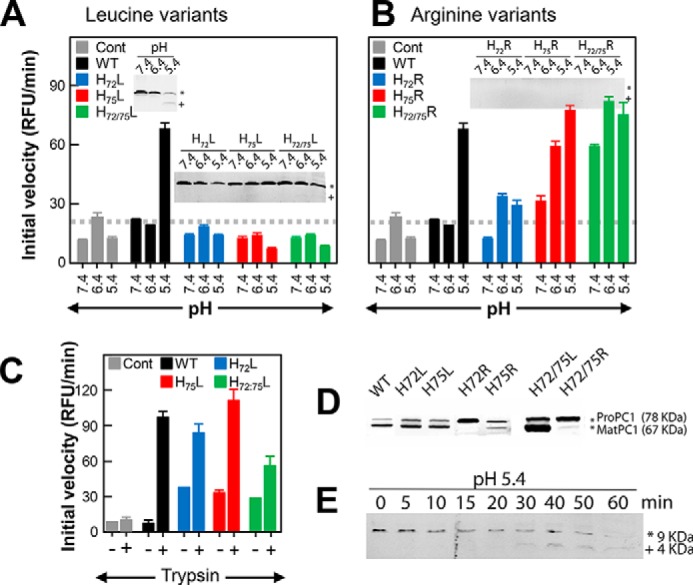
pH-dependent activation of the ER-retained precursor variants of PC1/3. Activity from ER extracts of cells transfected with KDEL-tagged pro-PC1/3 after preincubation at the indicated pH is shown. The activity profiles for WT precursor activated using various pH values are shown in black bars, variants at position 72 are shown in blue, at variants at position 75 are shown in red, and variants at both positions 72 and 75 are shown in green. Histidines at these positions were mutated to leucine (A) or arginine (B). Cells transfected with empty vector (Cont) were treated identically with the results shown by gray bars. Gray dashed lines indicate the threshold for baseline activity, below which an enzyme is considered inactive. Results are the mean ± S.D. (error bars) of three independent experiments performed in triplicate. Insets show Western blots detecting the product of the primary cleavage, which results in a 9-kDa fragment of PROPC1/3. The asterisk and plus indicate the uncleaved (∼9-kDa) and cleaved (∼4-kDa) forms of WT PROPC1/3 along with the leucine and arginine variants of His72 and His75. C, enzymatic activity using ER extracts from cells transfected with WT PROPC1/3 along with H72L-PROPC1/3, H75L-PROPC1/3, and H72L/H75L-PROPC1/3 after preincubation at pH 7.4 followed by incubation in the presence or absence of trypsin. D, Western blot showing the uncleaved precursor (pro-PC1/3; 78 kDa) and MATPC1/3 (67 kDa) for the WT and histidine variants. E, time course of processing of the WT PROPC1/3 after incubation at pH 5.4 for the indicated number of minutes. The asterisk and plus indicate the uncleaved (∼9-kDa) and cleaved (∼4-kDa) forms of PROPC1/3. RFU, relative fluorescence units.
