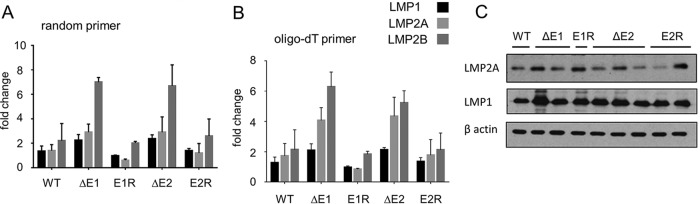
FIG 3.
Cytoplasmic RNA from two LCLs each for EBV wild type (WT), deletion of EBER1 (ΔE1), revertant (E1R), and deletion of EBER2 (ΔE2) or revertant (E2R) was used for cDNA synthesis performed with either random primers (A) or oligo(dT) (B) using a ProtoScript First Strand cDNA synthesis kit (New England BioLabs). Quantitative PCR (Q-PCR) performed with the same primers as those described in reference 10 was then used in duplicate assays to measure the levels of RNA for LMP1, LMP2A, and LMP2B, using GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a reference. The threshold cycle (2−ΔΔCT) method of comparative PCR (17) was used to analyze the results, expressed as fold change relative to the E1R LMP1 value. (C) Radioimmunoprecipitation assay (RIPA) lysates were prepared from LCLs, and equal amounts of cell protein were analyzed by Western immunoblotting. Membranes were probed with a 1/1,000 dilution of anti-LMP2A (Abcam; 14B7), a 1/500 dilution of anti-LMP1 (Dako; clone CS.1-4), or a 1/5,000 dilution of anti-β actin (Sigma; AC-74). Secondary antibodies were horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin (GE Healthcare) or horseradish peroxidase-conjugated rabbit anti-rat immunoglobulin (Sigma). Bound immunocomplexes were detected by enhanced chemiluminescence (GE Healthcare).