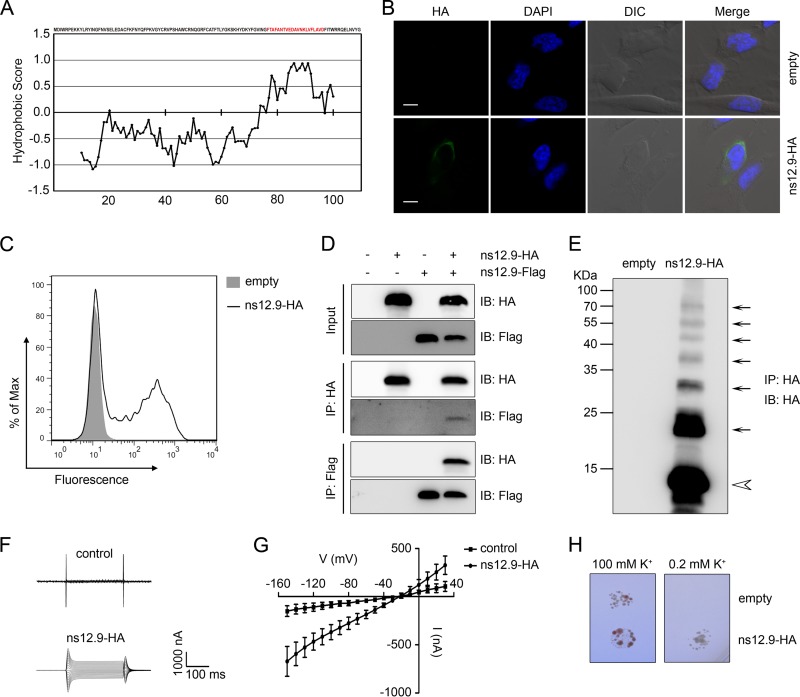
FIG 1.
ns12.9 accessory protein of HCoV-OC43 acts as a viroporin. (A) Kyte-Doolittle hydropathy plot of ns12.9 protein. The hydropathy plot was drawn with a window size of 19 using the Kyte-Doolittle method of hydrophilicity calculation. (B) Indirect immunofluorescence analysis of cell membrane expression of ns12.9 in RD cells transfected with pCAGGS-ns12.9-HA or empty vectors. Cells were fixed, nonpermeabilized, and subjected to incubation with anti-HA monoclonal antibody, followed by staining with Alexa Fluor 488-conjugated goat anti-mouse antibody (green). Nuclei were stained with DAPI (blue). Bars represent 15 μm. DIC, differential inference contrast. (C) Flow cytometry analysis of cell membrane expression of ns12.9 in 293T cells transfected with pCAGGS-ns12.9-HA or empty vectors. Cells were nonpermeabilized and stained with anti-HA monoclonal antibody, followed by Alexa Fluor 488-conjugated goat anti-mouse antibody. (D) Reciprocal coimmunoprecipitation assay of ns12.9-HA and ns12.9-Flag in 293T cells. Cells were transfected with pCAGGS-ns12.9-HA or pCAGGS-ns12.9-Flag vectors and lysed at 24 h posttransfection. Cell lysates were subjected to immunoprecipitation (IP) with anti-HA agarose or anti-Flag M2 affinity gel. The immunoprecipitated proteins were determined by Western blotting using polyclonal anti-Flag and anti-HA antibody. IB, immunoblot. (E) Oligomerization of the ns12.9-HA protein. The HA immunoprecipitates from cells transfected with pCAGGS-ns12.9-HA or empty vectors were analyzed by Western blotting with polyclonal anti-HA antibody. The monomer and oligomers are indicated with an arrowhead and arrows, respectively. Representative current traces (F) and I/V curve of the currents (G) in ns12.9-expressing and control oocytes are shown. During the current recording, the oocytes were bathed in ORi solution, and the standard voltage clamp protocol consisted of rectangular voltage pulses from −150 to +30 mV in 10-mV increments applied from a holding voltage of −60 mV. Data represent the means ± standard deviations (SD) (n = 5). (H) Complementation of the potassium uptake-deficient yeast. The yeasts transformed with empty or ns12.9-expressing vector were grown in parallel on plates supplemented with 100 mM or 0.2 mM KCl. Plates were kept at 30°C for 3 to 4 days.