FIG 3.
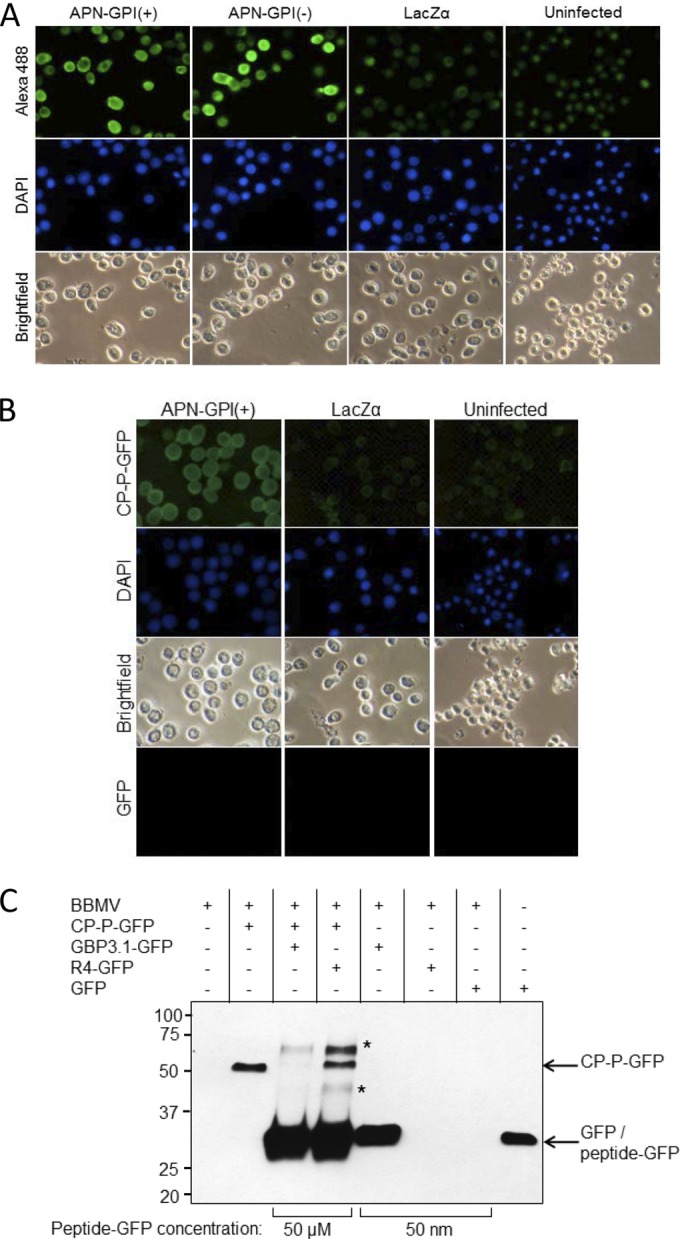
PEMV binding to APN is specific. (A) Baculovirus expression of recombinant APN in Sf9 cells. Sf9 cells were infected with the recombinant baculoviruses vAPN-GPI(+), vAPN-GPI(−), or vLacZα as a control, and expression of APN at 48 h was detected using an anti-APN primary antibody and an Alexa 488-conjugated secondary antibody. Nuclei were stained with DAPI. Uninfected cells served as a control. Alexa 488 (top row) and DAPI fluorescence (middle row) were visualized by epifluorescence microscopy. Bright-field images are also shown. The images are representative of four experiments. (B) Binding of CP-P-GFP to Sf9 cells expressing recombinant APN. Sf9 cells were infected with vAPN-GPI(+) or negative-control baculovirus, vLacZα, and at 48 h postinfection the cells were incubated with CP-P-GFP or GFP, followed by DAPI to stain the nuclei. Uninfected Sf9 cells served as an additional control. Increased fluorescence was observed in Sf9 cells expressing recombinant APN. No fluorescence was observed when cells were incubated with GFP. The images are representative of six experiments. Note that baculovirus infection results in enlargement of the nucleus, as seen for DAPI-stained, infected cells. All images were taken at the same magnification. (C) GBP3.1 competes with CP-P-GFP for binding to aphid BBMV. Shown is a Western blot with anti-GFP antiserum for the detection of GFP fusion proteins bound to BBMV in pulldown assays. The binding of CP-P-GFP (50 nM) was outcompeted by the addition of a 1,000-fold molar excess of GBP3.1-GFP (50 μM) but not by excess of the nonbinding control peptide R4-GFP. At 50 nM, GBP3.1-GFP showed strong binding to the BBMV compared to no observed binding of R4-GFP and GFP at the same concentration. R4-GFP was detected when used in excess (50 μM) but did not compete with the binding of CP-P-GFP (∼52 kDa). A total of 50 ng of GFP protein was used as positive control for the GFP antiserum. *, Proteins of ∼60 and 40 kDa detected after the addition of 50 μM peptide are hypothesized to be a dimer (∼57 kDa) and a degradation product of the peptide-GFP proteins, respectively. Conserved cysteines at positions 2 and 11 of the peptides provide for disulfide bonds which mediate dimerization at high concentration.
