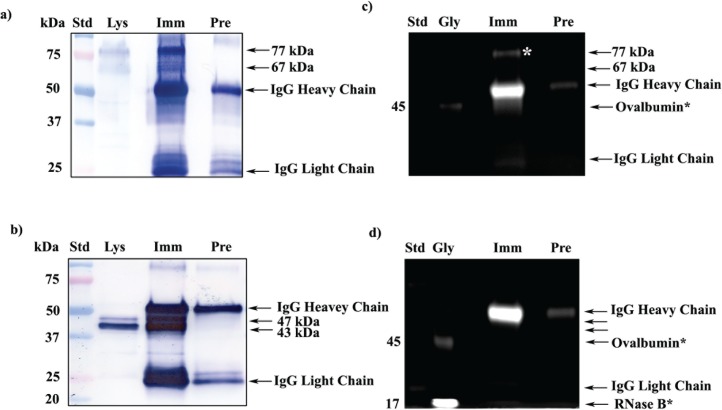
FIG. 3.
Glycoprofile staining of immunoprecipitated mouse testicular and sperm SPESP1. a) 1D Western blot probed with anti-SPESP1 serum shows 77- and 67-kDa SPESP1 isoforms in the starting testicular lysate (Lys). These isoforms were enriched in testicular immune complexes precipitated with immune (Imm) but not with nonimmune (Pre) sera. b) 1D Western blot of sperm lysate (Lys) revealed SPESP1 proteins at 47- and 43-kDa, which were enriched only in the immune, not in the nonimmune, immunoprecipitates. c) The 77-kDa testicular SPESP1 band (white asterisk) reacted with the glycoprofile stain only in the lane with immune serum precipitate. d) Neither the 47- nor the 43-kDa sperm band reacted positively with glycoprofile stain. c and d) In glycoprofile-stained gels, 56-kDa IgG heavy chains were stained in both immune and nonimmune lanes in accord with the known heavy-chain glycosylation at Asn297 [37]. Glycoprotein standards (Gly) ovalbumin at 45 kDa (c and d) and RNase at 17 kDa (d) were glycostain positive. e) SPESP1 isoforms immunoprecipitated from mouse testis were analyzed by 2D SDS-PAGE Western blot using SPESP1 antibody, revealing trains of SPESP1 charge variants at 77 and 67 kDa (red circle); 47 and 43 kDa (black circle). f) Several 77-kDa SPESP1 charge variants and one 67-kDa variant stained with glycoprofile. Nonimmune immunoprecipitates probed with SPESP1 immune serum showed only heavy and light chains (Supplemental Fig. S4). Std, Standard.