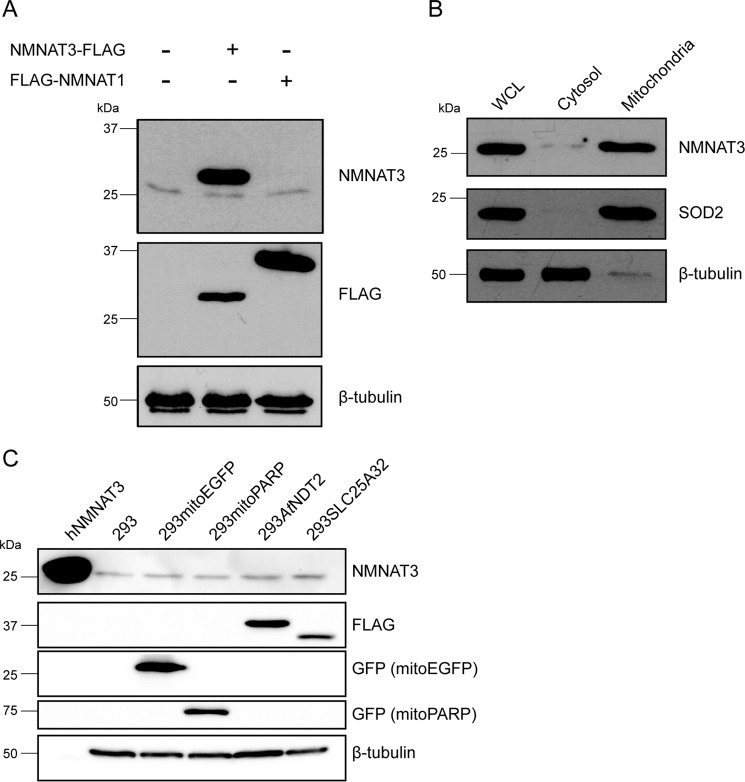
FIGURE 9.
NMNAT3 is present in mitochondria of human cells, and its expression level is unaffected by changes in the mitochondrial NAD+ content. A, Western blot detection of endogenous and overexpressed NMNAT3 in 293 cells with a monoclonal NMNAT3-specific antibody. The monoclonal antibody detected both the endogenous protein and the overexpressed FLAG-tagged recombinant NMNAT3. No cross-reactivity of the monoclonal antibody with NMNAT1 was observed. β-Tubulin served as a loading control. B, whole cell lysate (WCL), cytosolic and mitochondrial fractions from 293 cells were subjected to immunoblot analysis using the monoclonal NMNAT3-specific antibody. Superoxide dismutase (SOD2) served as a control for the purity of mitochondrial fraction, whereas β-tubulin served as a control for the cytosolic fraction. C, detection of endogenous NMNAT3 in lysates from 293 cell lines with altered mitochondrial NAD+ availability (293mitoPARP and 293AtNDT2), as well as control cells (293mitoEGFP and 293SLC25A32 and parental 293 cells). Lysates were subjected to immunoblot analysis using the NMNAT3-specific antibody. β-Tubulin served as a loading control. hNMNAT3, bacterially expressed, purified His6-tagged human NMNAT3 (47).