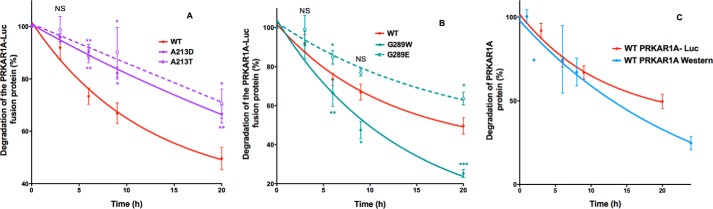
FIGURE 8.
Effect of missense mutations in the same amino acids of PRKAR1A causing either Carney complex or acrodysostosis on the degradation of PRKAR1A. HEK293 cells were transfected with PRKAR1A-luciferase constructs, plated in duplicate wells, and 24 h later, cycloheximide was added to half of the wells. At the indicated times after the addition of cycloheximide, luciferase activity was measured by luminometry. Results, expressed as the percentage of the time 0 fluorescence, are the mean ± S.E. (error bars) for at least three independent experiments, each performed in duplicate. Shown are findings for WT PRKAR1A (solid red lines) and PRKAR1A carrying mutations causing Carney complex (solid lines) and acrodysostosis (dotted lines) for the indicated mutations affecting Ala-213 (A) and Gly-289 (B). C, comparison of WT PRKAR1A degradation using Western blot or luminometry. HEK293 cells were transfected with WT PRKAR1A or PRKAR1A-luciferase constructs and plated in duplicate wells, and 24 h later, cycloheximide was added. At the indicated times after the addition of cycloheximide, the PRKAR1A protein was analyzed by Western blot (blue curve), or luciferase activity was measured by luminometry (red curve). Results, expressed as the percentage of results at time 0, are the mean ± S.E. for 3–5 independent experiments (unpaired Student's t test; *, p < 0.05; **, p < 0.01; ***, p < 0.001).