FIGURE 10.
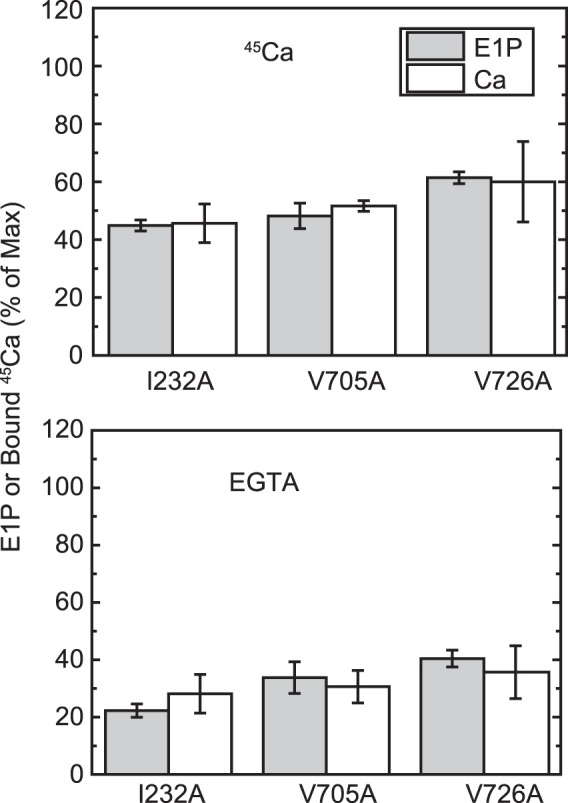
E1P fraction and bound Ca2+. The amount of bound Ca2+ and the E1P fraction in total amount of EP (E1P plus E2P) were determined repeatedly in the presence of an excess 1 mm EGTA (bottom panel) or 10 μm Ca2+ (top panel) or at one selected time point during the EP isomerization and Ca2+ release time courses (i.e. 2 s after the start of reaction) for the mutants I232A and V726A in comparison with the mutant V705A, as described in Fig. 6. The E1P fraction in the total amount of EP and the amount of bound 45Ca2+ relative to the maximum 45Ca2+ binding determined at zero time are shown as indicated (E1P and bound 45Ca, respectively). The values presented are the mean ± S.D. (n = 3–4). It should be noted that the Ca2+-ATPase is dephosphorylated to the E2 state upon Ca2+ removal by an excess of EGTA (bottom panel) and is in all phosphorylated states in the presence of 10 μm Ca2+ (top panel, see Figs. 6–8). Therefore, there is no Ca2+ bound to non-phosphorylated enzyme under our experimental conditions.
