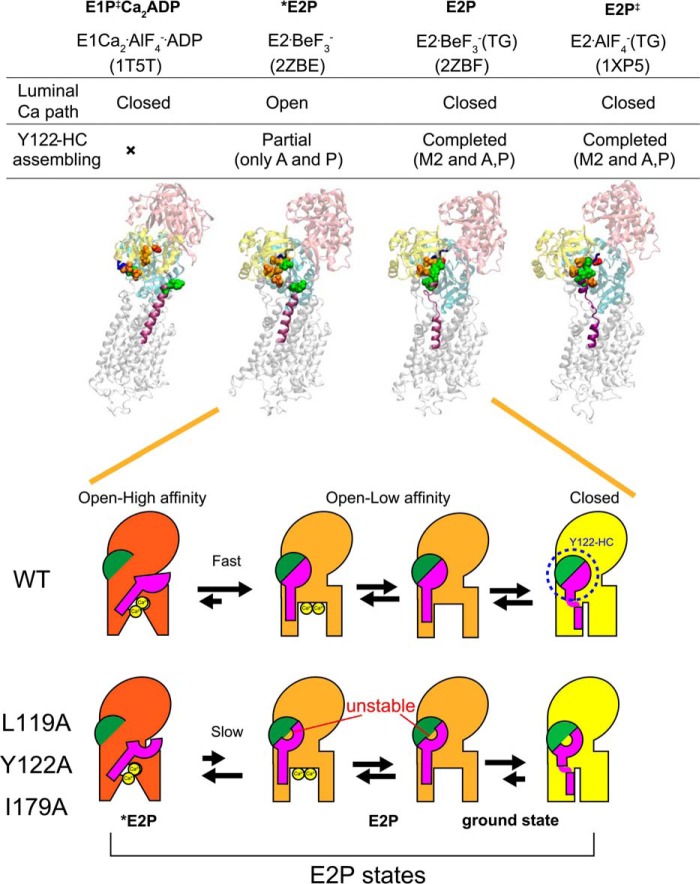
FIGURE 12.
Schematic for E2P processing and Ca2+ handling. Top panel, structures of E1Ca2·AlF4−·ADP (a structural analog for the transition state in phosphorylation, E1PCa2·ADP‡, PDB code 1T5T (Ref. 10)), E2·BeF3− (PDB code 2ZBE (Ref. 12)), E2·BeF3−(TG) (PDB code 2ZBF (Ref. 12)), and E2·AlF4−(TG) ((a structural analog for the transition state in hydrolysis, E2P‡, PDB code 1XP5 (Ref. 11)) are shown as a schematic. In these structures, the residues involved in the formation of the Tyr122 hydrophobic cluster (Y122-HC) are depicted with van der Waals spheres and are colored as in Fig. 10 (see the residue numbers in Fig. 10). The cytoplasmic part of M2 and the TGES184 loop are colored in purple and blue, respectively, and the A, P, and N domains are colored in yellow, cyan, and pink, respectively. The open or closed state of the Ca2+ path (luminal gate) and the assembling state of the Tyr122 hydrophobic cluster are indicated above the structures. Bottom panel, schematic of the structural changes for Tyr122 hydrophobic cluster formation and for the property of the Ca2+ transport sites and release gate during E2P processing with Ca2+ release (E2PCa2 to E2P). In this model, the main body of Ca2+-ATPase is shown in red, orange, and yellow to indicate the open high-affinity, open low-affinity, and closed states, respectively, for the property of the Ca2+ transport sites and release gate. The green semicircle indicates the part of Tyr122 hydrophobic cluster formed in E2PCa2 upon EP isomerization (E1PCa2 → E2PCa2) and composed of Ile179/Leu180 on the A domain, Val705/Val726 on the P domain, and Ile232 on the A/M3 linker. The pink structure indicates the cytoplasmic part of M2, including Leu119 and Tyr122. During E2P processing, Tyr122/Leu119 gathers to Ile179 and then to the assembled five residues, completing the Tyr122-hydrophobic cluster, which is coupled with affinity reduction. Mutation of Leu119, Tyr122, or Ile179 probably destabilizes the hydrophobic cluster, thereby retarding affinity reduction. Also note that our previous detailed kinetic study has revealed (18) that the E2P ground state structure has a closed luminal gate and that the Tyr122 hydrophobic cluster is tightly fixed (as described under “Discussion”), which is depicted here with a yellow body.