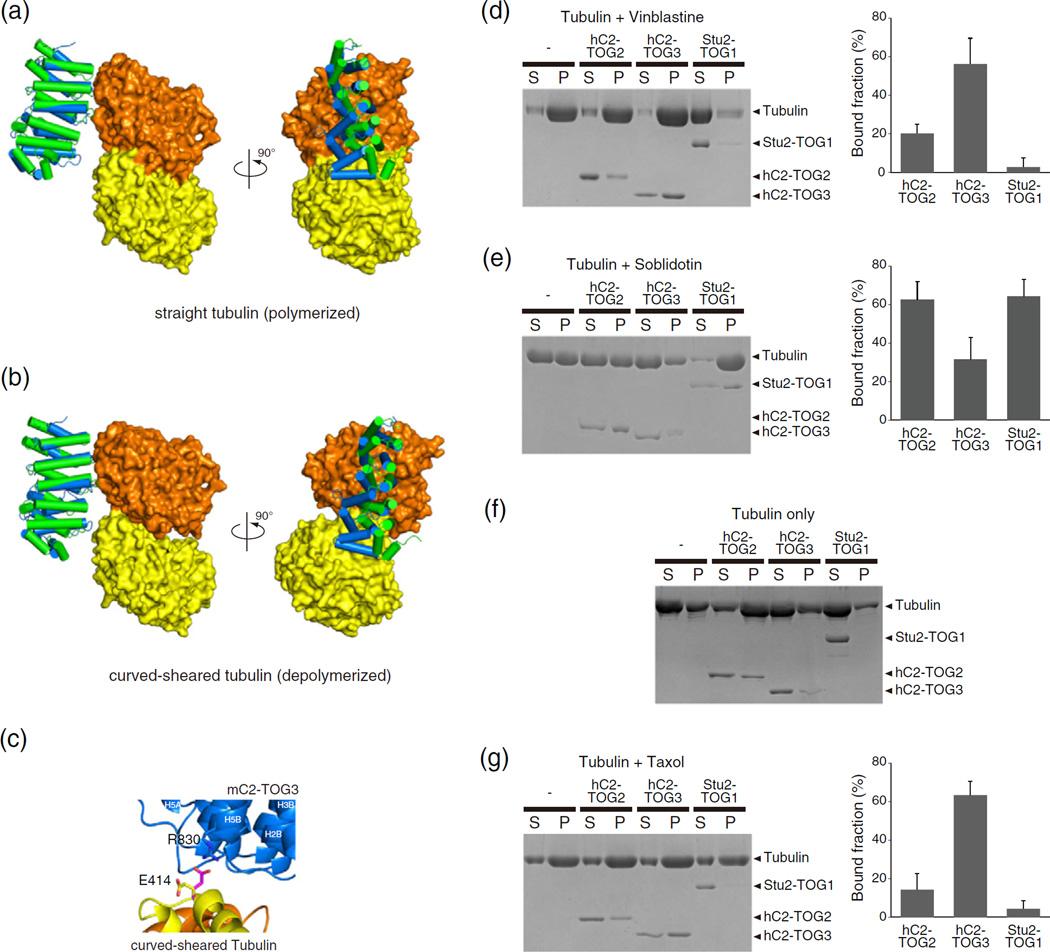
Fig. 6.
TOGs and tubulin interactions. (a and b) The hypothetical models of the complexes of αβ-tubulin with CLASP2-TOGs. The models of CLASP2-TOGs with straight tubulin in the MT lattice (a) and depolymerized curved-sheared tubulin (b) are shown. All colors are the same as in Fig. 5a. (c) Close-up view of the interaction site of the hypothetical model between mC2-TOG3 and curved-sheared αβ-tubulin. Cartoon models of mC2-TOG3, α-tubulin and β-tubulin are colored blue, yellow and orange, respectively. A conserved residue in HR5, R830, can make a contact with the side chain of E414 in α-tubulin. The side chain of E414 in the crystal structure is colored yellow, and the same residue in the hypothetical model is shown in magenta. The nitrogen and oxygen atoms in the side chain are colored blue and red, respectively. The orientation is related to that in the right panel of (c) by a 90° rotation about a horizontal axis (Supplementary Fig. S6). (d–g) (left) SDS-PAGE of tubulin co-sedimentation assays with TOGs. Vinblastine-treated tubulin (d), soblidotin ring (e), tubulin with 0.1 mM GTP (f) and taxol-stabilized MTs (g), without TOG, with hC2-TOG2, hC2-TOG3 and Stu2-TOG1 are shown. S, supernatant; P, pellet. Without tubulin, all TOG domains were not sedimented (Supplementary Fig. S7). (d, e and g) (right) Quantification of MT co-sedimentation assays. Error bars represent standard deviations (n = 3).