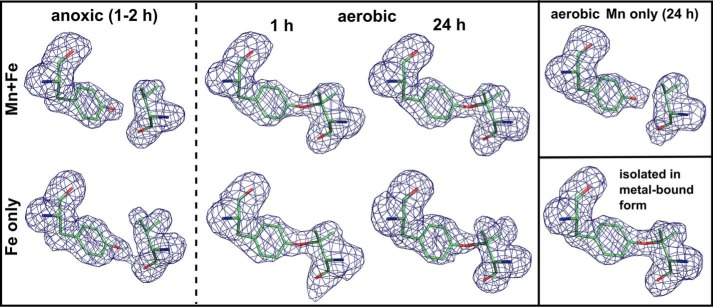
FIGURE 7.
Tyrosine-valine ether cross-link formation catalyzed by different metal cofactors in R2lox. mFo − DFc omit electron density for residues Tyr-162 and Val-72 in apoprotein crystals soaked with manganese and/or iron in the absence or presence of oxygen for the indicated durations, and in protein isolated in metal-bound form, contoured at 3.0 σ. The cross-link is absent when the cofactor is reconstituted in the absence of oxygen, whereas in the presence of oxygen it is formed by both the Mn/Fe and the Fe/Fe cofactor, but not the Mn/Mn cluster. For clarity, the link is modeled here where the density shows it is present, but poor density fits and too long refined bond lengths indicate it is only partially formed in soaked apoprotein crystals. Quantification of the cross-link by mass spectrometry shows that it is formed equally efficiently by the Mn/Fe and the Fe/Fe cofactor (data not shown).