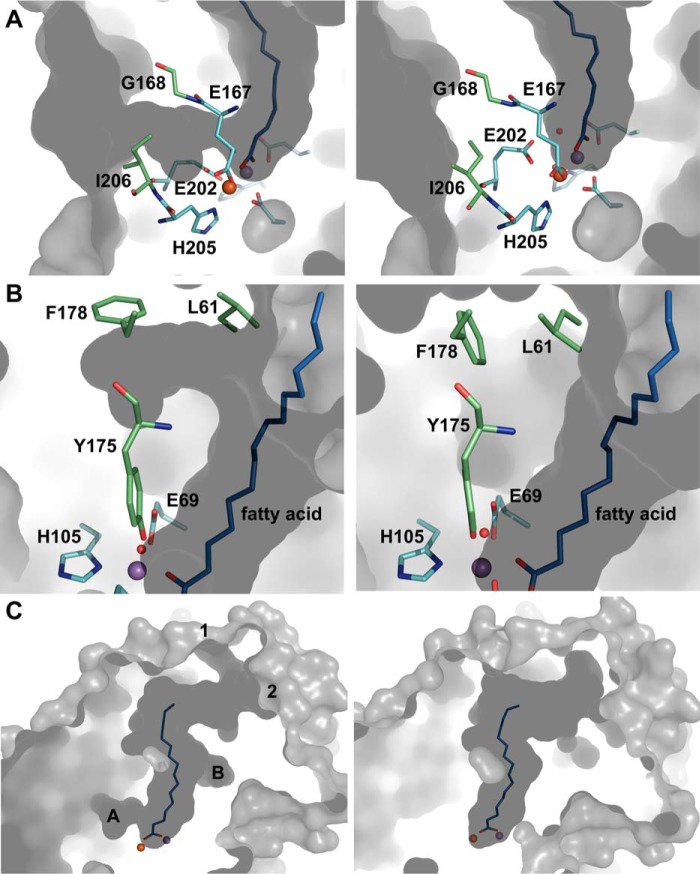
FIGURE 8.
Likely routes for gated oxygen and substrate access to the active site. A, residues Glu-202 and Ile-206 of R2lox gate a hydrophobic channel leading from the protein surface to metal site 2. Rotamer changes open the channel in the reduced state (left) and close it in the oxidized state (right). B, residues Leu-61 and Phe-178 also adopt different rotamers in the reduced (left) and oxidized (right) state, creating a pocket branching off of the ligand-binding tunnel in the reduced state. C, two entrances to the ligand-binding pocket are gated by a flexible loop. They are open in the reduced Mn/Fe state (left) but occluded in the oxidized Mn/Fe state (right), whereas in the other metallation states they are found in various combinations of open and closed (data not shown). The tunnel entrances are labeled 1 and 2, and the pockets/channels shown in A and B are indicated. The same color scheme is used as in Fig. 2. Shown in gray is the molecular surface of R2lox, as calculated by CASTp (106) using a 1.4 Å probe radius.