FIGURE 3.
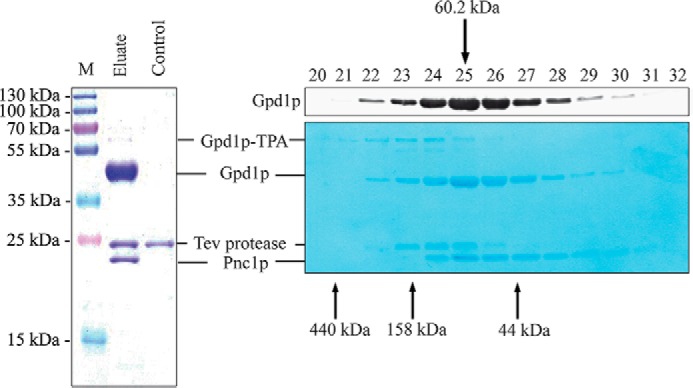
Pnc1p associates with Gpd1p. Gpd1p was affinity-purified from soluble fractions of a strain expressing Gpd1p-TPA under control of its own promoter. Cells were grown on high salt-containing medium and disrupted by glass bead breakage. Soluble fractions were applied to affinity chromatography at an IgG matrix, and Gpd1p-associated proteins were eluted by TEV protease cleavage (left panel). Wild-type cells without genomically integrated TEV-protein A served as a negative control. Gpd1p-associated proteins were separated by SDS-PAGE, and Coomassie-stained polypeptides were identified by mass spectrometry. M, molecular mass marker. Gpd1p complexes were further fractionated by calibrated size-exclusion chromatography (right panels). Fractions were collected, subjected to immunoblot analysis, and either stained with Amido Black dye (lower right panel) or immunodetected using antibodies against Gpd1p (upper right panel). Molecular masses of calibration proteins and calculated mass of the Gpd1p-Pnc1p complex are indicated.
