FIGURE 2.
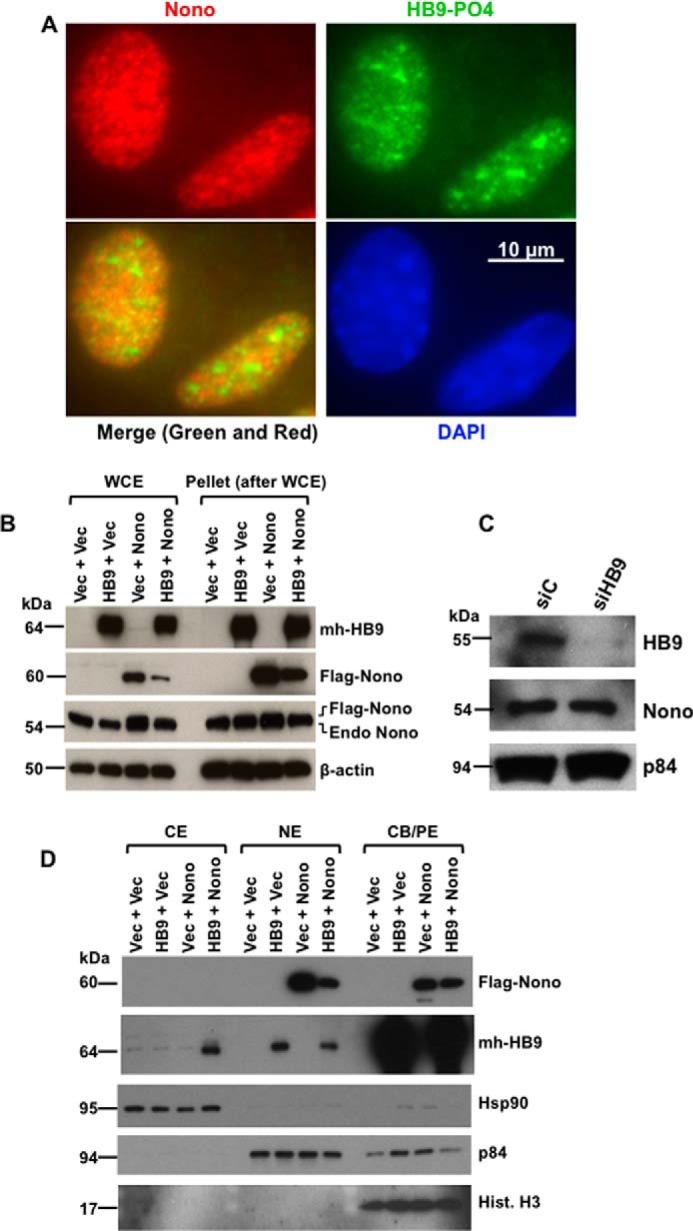
HLXB9 co-localizes with Nono in the nucleus, and co-overexpression of HLXB9 and Nono decreases the overexpression of Nono with translocation of HLXB9 into the cytoplasm. A, endogenous phospho-HLXB9 co-localizes with endogenous Nono in the nucleus. IF images of MIN6-4N cells show endogenous Nono (red) and phospho-HLXB9 (green). DAPI was used to detect the nuclei (blue). A merged image of the red and green IF shows co-localization of Nono and phospho-HLXB9 in subnuclear spots and some regions with phospho-HLXB9 (green) that did not co-localize with Nono. B, overexpression of HLXB9 decreases the level of overexpressed Nono protein. Western blots are shown of WCE and the pellet leftover after WCE preparation from MIN6-4N cells expressing mh-HB9-WT, FLAG-Nono, or both together. Empty vector DNA (Vec) was used to maintain the same amount of DNA in the transfections. The expression of transfected HLXB9 was detected with the anti-myc-tag; Nono was detected with the anti-FLAG-tag and with anti-Nono to detect both endogenous and transfected FLAG-tagged Nono. β-Actin was used as the loading control. Endogenous Nono levels were not affected by HLXB9 overexpression. However, the level of transfected FLAG-Nono was reduced upon HLXB9 overexpression. A similar pattern of bands was seen in the pellet leftover after WCE preparation, indicating that the reduced level of Nono upon HLXB9 overexpression was not due to differential cell lysis in the WCE preparation. C, HLXB9 did not reduce the expression of endogenous Nono protein. Western blot analysis to detect endogenous HLXB9 and Nono using WCE prepared from MIN6-4N cells transfected with control siRNA (siC) or HLXB9 siRNA (siHB9) is shown. p84 was used as the loading control. HLXB9 was significantly knocked down, but that did not affect the level of endogenous Nono. D, co-overexpression of HLXB9 and Nono reduces the level of Nono protein in the nucleus with translocation of HLXB9 to the cytoplasm. Shown is Western blot analysis of subcellular fractionation of CE, NE, and CB/PE from MIN6-4N cells expressing mh-HB9-WT, FLAG-Nono, or both together. Empty vector DNA (Vec) was used to maintain the same amount of DNA in the transfections. The expression of transfected HLXB9 was detected with the anti-myc-tag; Nono was detected with the anti-FLAG-tag; detection of marker proteins (Hsp90 for CE, p84 for NE, and histone H3 for CB/PE) showed minimal cross-contamination of the fractions and also served as loading controls for each fraction. Overexpressed Nono was found in the NE and in the CB/PE, but its level in NE was reduced by HLXB9 overexpression. Overexpressed HLXB9 was mostly located in the CB/PE and with a significant amount in the nucleus, but it was also detected in the CE by Nono overexpression.
