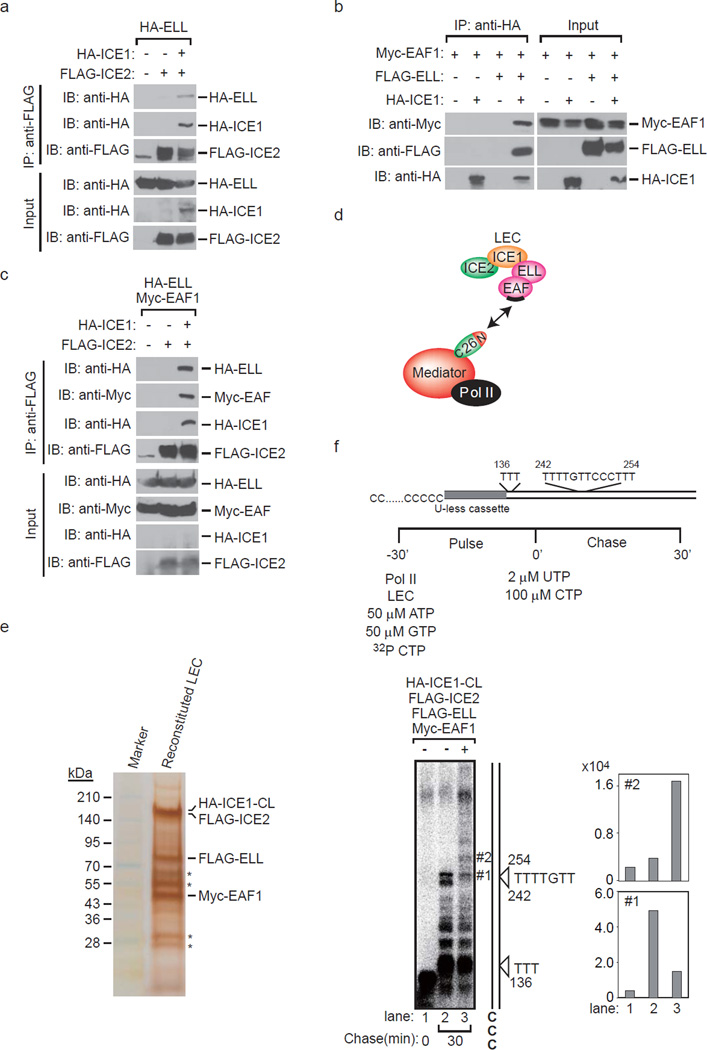
Figure 2. Reconstitution of little elongation complex by a baculovirus expression system.
(a) ICE2 (NARG2) binds to ELL in the presence of ICE1 (KIAA0947). FLAG-immunopurified complexes from baculovirus-infected Sf9 cells expressing the indicated proteins were analyzed by Western blotting. (b) ICE1 binds to EAF1 in the presence of ELL. HA-immunopurified complexes from baculovirus-infected Sf9 cells expressing the indicated proteins were analyzed by Western blotting. (c) ICE2 binds to ELL/EAF1 in the presence of ICE1. FLAG-immunopurified complexes from baculovirus-infected Sf9 cells expressing the indicated proteins were analyzed by Western blotting. (d) Proposed architecture of the components of LEC and model for MED26 NTD function as a docking site for LEC. MED26 NTD interacts with LEC through direct interaction with EAF. In turn, the MED26 C-terminal domain binds to Pol II through Mediator. The binding site for MED26 NTD on EAF is represented by black bars. (e) Silver staining of HA-ICE1-CL/FLAG-ICE2/FLAG-ELL/Myc-EAF1 complex reconstituted by the baculovirus expression system. FLAG-ELL, Myc-EAF1, FLAG-ICE2 and HA-ICE1 C-terminal fragment (CL: 1191–2266) were co-expressed in Sf9 insect cells, and the ICE1-CL/ICE2/ELL/EAF1 complex was purified by anti-HA affinity chromatography as described in the Methods section. * indicates heavy chain and light chain derived from anti-HA antibodies used in affinity purification. (f) ICE1-CL/ICE2/ELL/EAF1 complex enhances transcription elongation by Pol II. Oligo(dC)-tailed template transcription assays were performed as described in the Results and Methods sections. Arrowhead indicates the position of the nascent transcript that is synthesized in the presence of ATP, GTP and CTP and stalled at the T site.