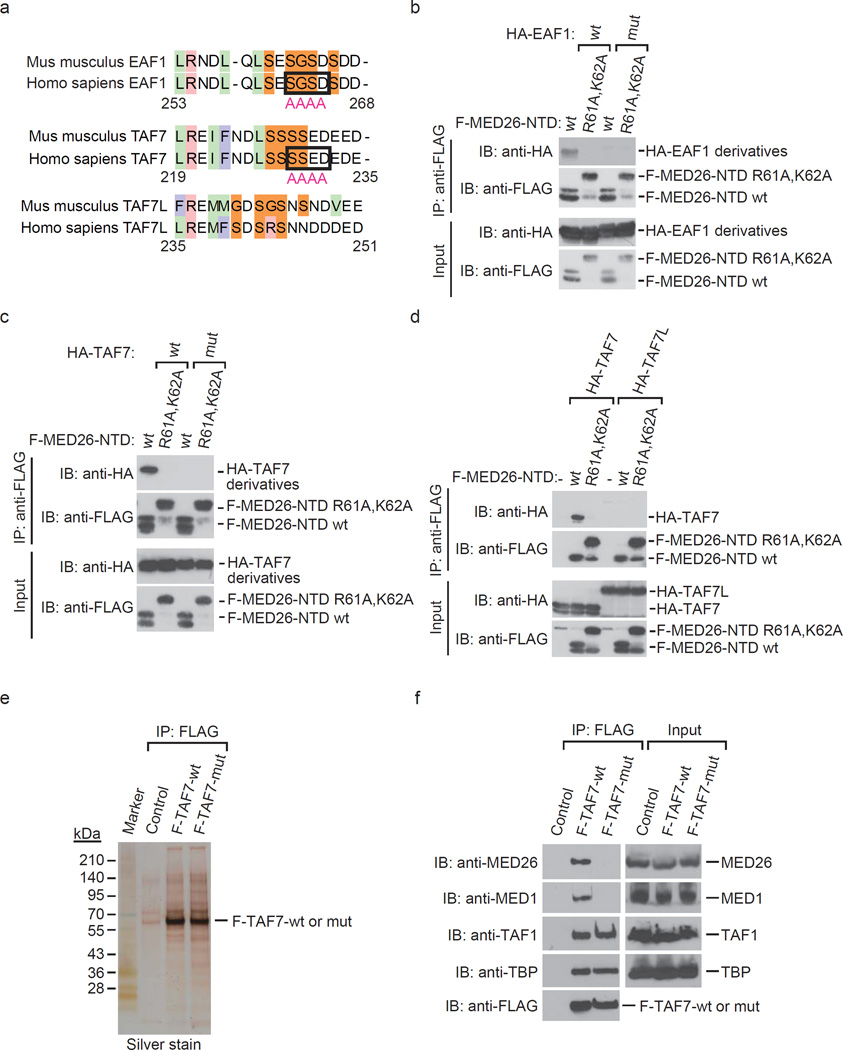
Figure 7. Identification of TAF7 and EAF1 mutations that interfere with MED26 NTD binding.
(a) Sequence alignment of EAF1, TAF7 and TAF7L in amino acid sequence that is required for interaction with MED26 NTD. Light green indicates hydrophobic residues. Pink column indicates basic residues. Light blue column indicates aromatic residues. Orange column indicates neutral residues. The residues which are substituted for alanine in order to generate mutants of EAF1 and TAF7 are surrounded by square. The indicated number of amino acids is from human EAF1 and TAF7, respectively. (b) Substitution of S262, G263, S264 and D265 to A interferes with human EAF1 interaction with MED26 NTD. Recombinant proteins of wild type (wt) or point mutant (R61A,K62A) of FLAG-tagged MED26-NTD were immobilized on anti-FLAG M2 agarose. The M2 agarose complex was incubated with recombinant proteins of HA-tagged EAF1 wild type (wt) or point mutant (mut). After washing, bound proteins were eluted and analyzed by Western blotting. (c) Substitution of S229, S230, E231 and D232 to A interferes with human TAF7 interaction with MED26 NTD. Recombinant proteins of wild type (wt) or point mutant (R61A,K62A) of FLAG-tagged MED26-NTD were immobilized on anti-FLAG M2 agarose. The M2 agarose complex was incubated with recombinant proteins of HA-tagged TAF7 wt or point mutant (mut). After washing, bound proteins were eluted and analyzed by Western blotting. (d) TAF7L does not bind to MED26 NTD. Recombinant proteins of wild type (wt) or point mutant (R61A,K62A) of FLAG-tagged MED26-NTD were immobilized on anti-FLAG M2 agarose. The M2 agarose complex was incubated with recombinant proteins of HA-tagged human TAF7 or HA-tagged mouse TAF7L. After washing, bound proteins were eluted and analyzed by Western blotting. (e) Silver staining of TFIID purified from HeLa cells stably expressing FLAG-tagged TAF7 wild type (wt) and point mutant (mut) by anti-FLAG affinity chromatography. (f) Western blotting for FLAG-immunopurified complexes from parental HeLa cells (control) and HeLa cells stably expressing FLAG-tagged TAF7 wild type (wt) and point mutant (mut).