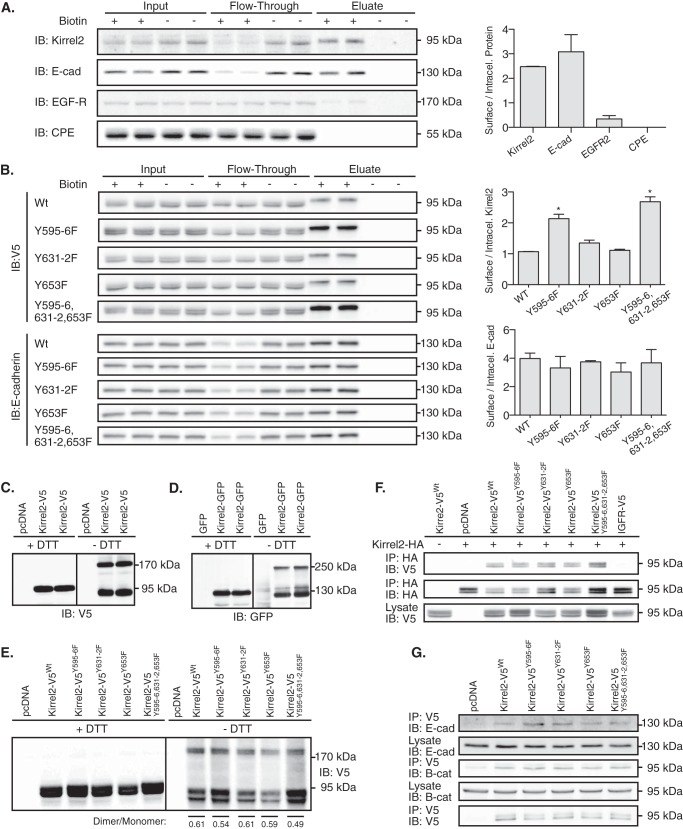
FIGURE 6.
Kirrel2Y595F/Y596F stabilization at plasma membrane is independent of its dimerization. A, intact MIN6 cells treated with or without Sulfo-NHS-SS-Biotin were processed according to the kit instructions. Collected fractions, including input, flow-through, and eluate, were analyzed by immunoblotting (IB) with antibodies against Kirrel2, E-cadherin (E-cad), EGFR, and CPE. Ratios of biotinylated (surface) to unbiotinylated (intracellular) proteins based on densitometry of the immunohybridization signals are shown in the right panel. B, MIN6 cells, transfected with V5-tagged wild type or mutant Kirrel2 expression plasmids, were treated as described in A. Collected fractions were analyzed by immunoblotting with anti-V5 (upper panel) and with anti-E-cadherin antibodies (lower panel). Ratios of biotinylated (surface) to unbiotinylated (intracellular) proteins are shown in right panels. *, p < 0.05 at Student's t test. C and D, MIN6 cells were transfected with Kirrel2-V5 or Kirrel2-GFP expression plasmids together with their respective controls. Protein lysates were resolved with reducing (+DTT) or non-reducing (−DTT) SDS-PAGE and blotted with anti-V5 or -GFP antibodies. E, Western blot of MIN6 cell lysates expressing the indicated Kirrel2-V5 proteins, resolved with reducing (+DTT) and non-reducing (−DTT) SDS-PAGE following immunoblotting with anti-V5 antibodies. F, lysates of MIN6 cells co-expressing wild type Kirrel2-HA and mutant Kirrel2-V5 were incubated with anti-HA affinity gel; immunoprecipitates were immunoblotted with anti-V5 and anti-HA antibody. A total of 50 μg of protein was immunoblotted with anti-V5 antibodies as input control. G, anti-V5 affinity gel immunoprecipitates of MIN6 cell lysates transiently expressing V5 tagged wild type or mutant Kirrel2 immunoblotted with anti-E-cadherin, -β-catenin, and -V5 antibodies.