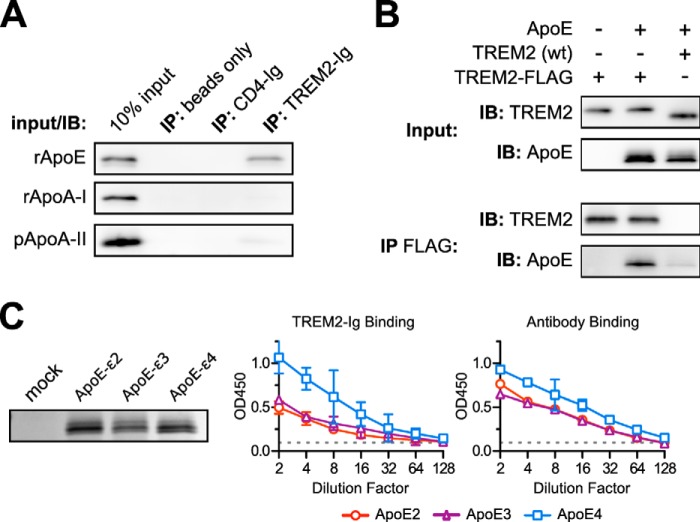
FIGURE 4.
TREM2-Ig binds ApoE specifically and irrespective of genotype. A, TREM2-Ig or control beads were used to immunoprecipitate (IP) solutions of the indicated purified apolipoproteins. Shown here are the results of SDS-PAGE and Western blots for each of the indicated apolipoproteins. The leftmost lane in each blot contains a sample of the input as a positive control. These results are representative of three independent experiments. B, ApoE co-immunoprecipitates (IP) with C terminally FLAG-tagged TREM2 when both constructs are co-transfected into HEK293T cells. Cells were co-transfected with three different plasmid mixtures as indicated at the top of the figure. The top two blots (Input) show immunostaining of the clarified lysates and the bottom two blots (IP FLAG) show staining of the corresponding immunoprecipitates. The left lane demonstrates the specificity of the ApoE antibody. The middle lane shows that FLAG-tagged TREM2 bound to ApoE, whereas the right lane includes an untagged, rather than FLAG-tagged, TREM2 as a negative control. The faint ApoE band in the rightmost lane of the IP FLAG blot shows the level of background binding of ApoE to the Sepharose support of the anti-FLAG beads. Recovery of ApoE from cell lysates was generally inefficient compared with other experiments so the (IP FLAG) ApoE blot was exposed 5 times longer than the corresponding (Input) ApoE blot. These results are representative of three independent experiments. C, ApoE2, E3, and E4 were expressed in HEK293T cells. Levels of ApoE in culture supernatants were approximately equivalent as shown by SDS-PAGE and Coomassie staining (left). A dilution series of these supernatants were used as immobilized ligands for ELISA. The left graph shows the binding of TREM2-Ig to each of the ApoE variants, whereas the right graph shows the binding of a polyclonal control serum. Error bars show 95% confidence intervals for triplicate wells. Similar results were obtained with a second preparation of ApoE (not shown).