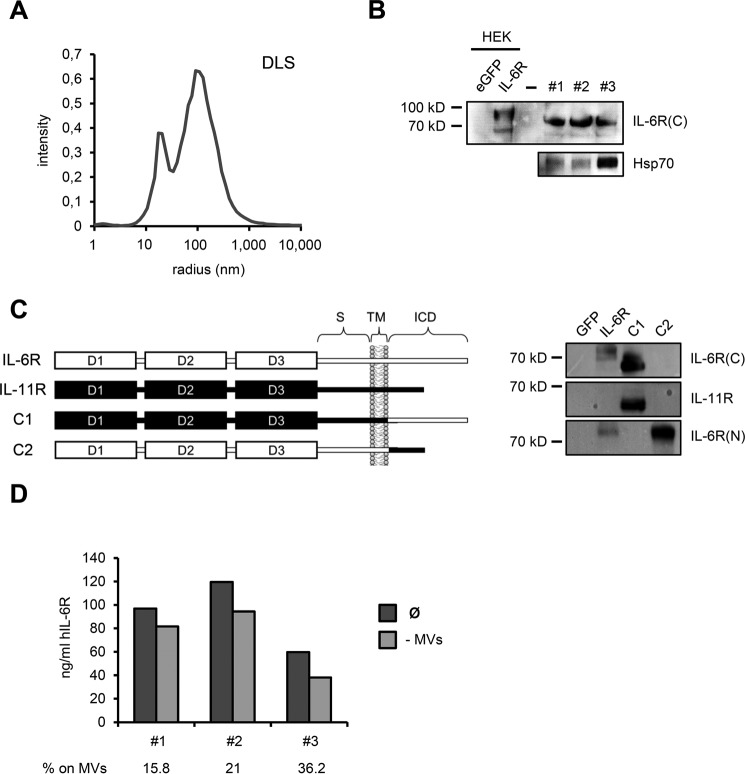
FIGURE 6.
A, microvesicle suspension was analyzed by DLS measurements and revealed two predominant fractions with hydrodynamic sizes of 19 and 100 nm. Whereas an average size of 100 nm is consistent with exosomes, the smaller fraction at 19 nm most likely corresponds to vesicle fragments resulting from the microvesicle enrichment procedure. B, Western blot analysis showing full-length IL-6R on circulating microvesicles of three healthy individuals, with the employed antibody specifically recognizing the intracellular C terminus of human IL-6R. cDNAs encoding GFP and human IL-6R were transiently transfected into HEK293 cells and served as negative and positive controls, respectively. Note that microvesicle-associated IL-6R appeared slightly smaller than cellular IL-6R in HEK293 lysates. Circulating microvesicles also contained substantial amounts of exosomal marker protein Hsp70. C, left: schematic showing composition of human IL-6R/IL-11R chimeras used to confirm antibody specificity. Portions of human IL-6R are depicted in white, whereas human IL-11R parts are highlighted in black. S, stalk region; TM, transmembrane region; ICD, intracellular domain. Construction of IL6R/IL-11R chimeras was described previously (54). Right: HEK cells were transiently transfected with IL-6R/IL-11R chimeras, along with GFP as negative control. IL-6R-transfected HEK cells served as positive control. The antibody used in this study to detect microvesicle-associated IL-6R only detects the human IL-6R C terminus, but not the IL-11R intracellular portion. IL-6R(C), antibody recognizing intracellular IL-6R C terminus; IL-6R(N), antibody against extracellular IL-6R N terminus. D, serum of the same individuals as in B was subjected to ultracentrifugation and sIL-6R levels prior and after ultracentrifugation were quantified by ELISA. Serum after ultracentrifugation was regarded as microvesicle-free (−MVs). Proportion of circulating sIL-6R associated with microvesicles is depicted below the diagram in %.