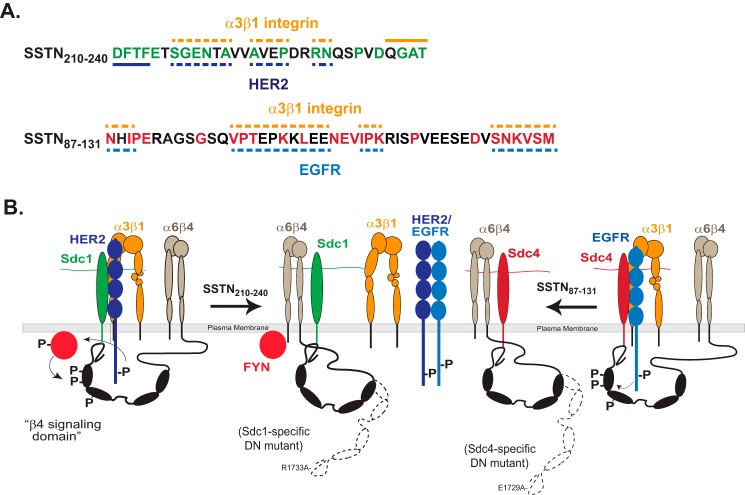
FIGURE 9.
Model of SSTN peptides and receptor interactions. A, SSTN210–240 and SSTN87–131 are shown, along with highlighted homology sequences and potential binding sites for α3β1 integrin, HER2 or EGFR. Solid line denotes binding site, whereas dashed line denotes site of possible interaction. B, model showing association of HER2, α3β1 integrin and α6β4 integrin with Sdc1, and their displacement by SSTN210–240. The β4 subunit is phosphorylated in its “signaling domain” by Fyn kinase when bound to Sdc1 and HER2 is activated by clustering of the receptor complex (3). The engagement of α6β4 integrin with Sdc1 is prevented by mutation of Arg-1733 in the β4 cytoplasmic domain, which acts as a dominant negative mutant to blocks the HER2-dependent mechanism, as shown by Wang et al. (2). The model also depicts the association of EGFR, α3β1 integrin, and α6β4 integrin with Sdc4, and their displacement by SSTN87–131. The engagement of α6β4 integrin with Sdc4 is prevented by mutation of E1729A in the β4 cytoplasmic domain, which acts as a dominant negative mutant to block the EGF-stimulated mechanism (2). The model also depicts the heterodimerization of HER2 and EGFR, which is not affected by SSTN peptides. EGFR homodimerization, or heterodimerization with other family members is not shown.