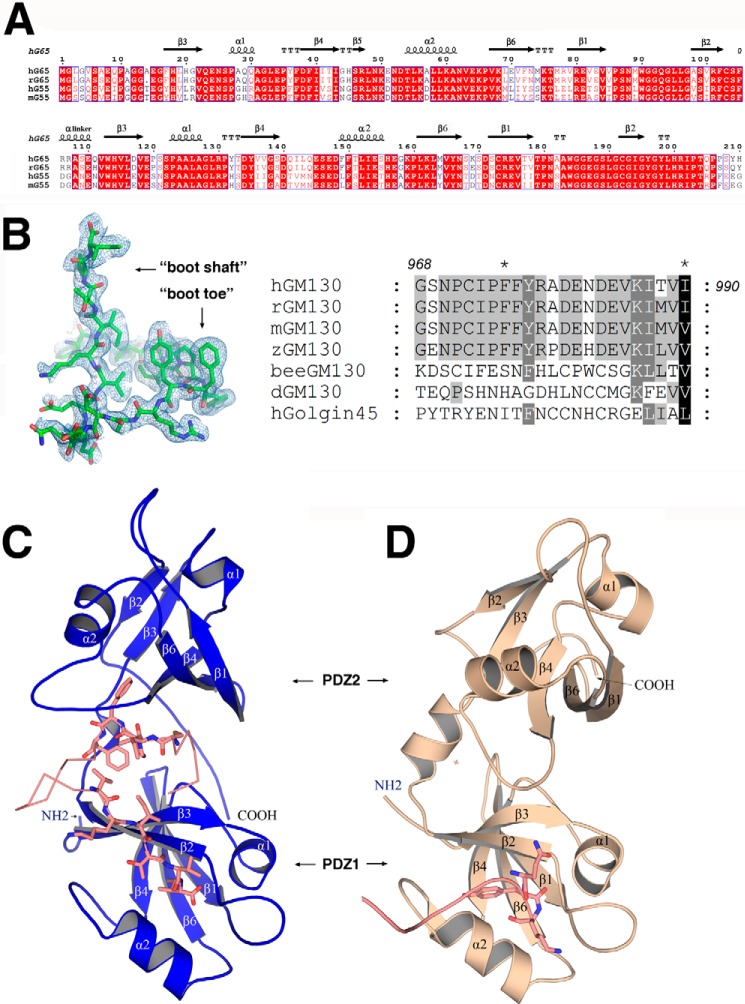
FIGURE 1.
Sequence alignment and crystal structures of GRASP65. A, sequence alignment of GRASP domains from human (hG65, NP_114105; hG55, Q9H8Y8.3), rat (rG65, AAI61850.1), and mouse (mG55, NP_081628.3). The strands are numbered according to their GRASP55 PDZ domain structure (27). The figure was generated with ESPript (47). B, electron density map of the bound GM130 C-terminal peptide (left) and a sequence alignment of GM130 from human (AAF65550.1), rat (NP_072118.2), mouse (NP_598613), zebra fish fish (XP_005168139.1), honey bee(XP_623918.3), and Drosophila (CAB77666.1) as well as human GOLGIN45 (NP_003657.1) C-terminal peptides (right). C, schematic representation of the overall structure of GRASP65 (colored blue) bound with the GM130 C-terminal peptide (colored salmon). D, schematic representation of the unbound structure of GRASP65 (Protein Data Bank code 4KFV, colored wheat) illustrating the neighboring molecular C terminus (colored salmon) interacting with the PDZ1 classic peptide binding groove.