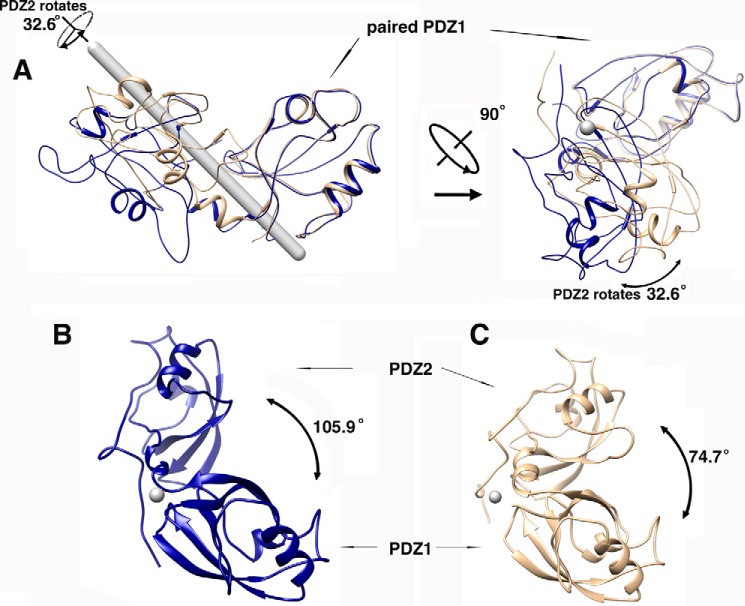
FIGURE 2.
Conformational changes of GRASP65 upon GM130 C-terminal peptide binding. The software program Chimera was used for superimposing the structures, measuring the angles of rotation of the domains and generating the figures. The gray rod denotes the rotation axis parallel to the paper plane, and the gray spots denote the rotation axis perpendicular to the paper plane. A, the superimposed structures of GRASP65 with and without the GM130 C-terminal peptide using the PDZ1 domain to pinpoint a rotation of 32.6 degrees at the PDZ2 along its axis. The two representations are rotated by 90 degrees. The unbound GRASP65 structure is colored wheat; the bound GRASP65 structure is colored blue. B, measurement of the angle between the PDZ1 and PDZ2 domains of GRASP65 upon GM130 binding. C, measurement of the angle between the PDZ1 and PDZ2 domains of GRASP65 without GM130 binding.