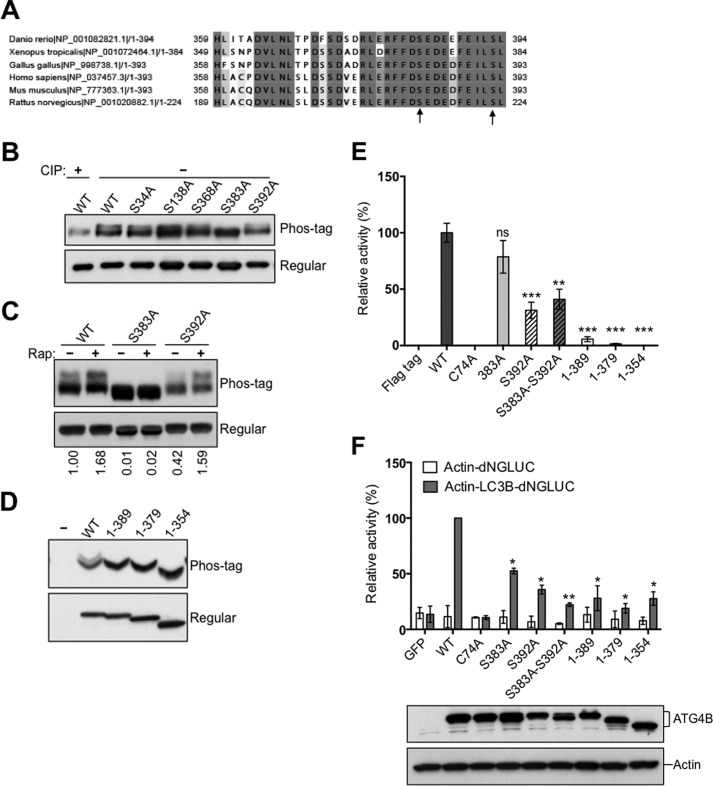
FIGURE 3.
Phosphorylation of the C-terminal region of ATG4B. A, protein sequence alignment of the C-terminal region of ATG4B in zebrafish (Danio rerio), frog (Xenopus tropicalis), chicken (Gallus gallus), human (Homo sapiens), mouse (Mus musculus), and rat (Rattus norvegicus) using the ClustalW2 program. Gray shading indicates the identities of residues among proteins of different species, generated by the Percentage Identity option in Jalview, with darker gray signifying identity in more species. Amino acid residues known to become phosphorylated in mammalian cells are shown by arrows. B, Flag-ATG4B WT and various mutants (as labeled) were expressed in HEK293T cells. Cell lysates were prepared and normalized for total protein content. Flag-tagged ATG4B was then immunoprecipitated and treated in vitro with or without CIP. Flag-ATG4B proteins were fractionated by phos-tag versus regular SDS-PAGE then analyzed by immunoblotting using anti-ATG4B antibody. C, HEK293T cells expressing Flag-ATG4B WT were cultured with or without rapamycin for 5 h. Cell lysates were subjected directly to phos-tag versus regular SDS-PAGE and immunoblotting was performed using anti-ATG4B antibody. The fold increase in the ratio of phosphorylated/non-phosphorylated ATG4B before and after rapamycin treatment was quantified using scanning densitometry (values shown below lanes). D, full-length Flag-ATG4B and various C-terminal-truncated mutants were expressed in HEK293T cells. Cell lysates were fractionated by phos-tag versus conventional SDS-PAGE and analyzed by immunoblotting using anti-ATG4B antibody. E, protease activity of immunoprecipitated Flag-ATG4B WT or various mutants was measured using the LC3-PLA2 assay over time (0–80 min). ATG4B activity at 30 min was within the linear phase of the reaction and was normalized relative to ATG4B WT (set as 100%; mean ± S.D.; n = 3; **, p < 0.01; ***, p < 0.001.). F, ATG4B Ser-383 and Ser-392 residues are important for LC3 cleavage in vivo. Actin-LC3B-dNGLUC (the reporter plasmid for LC3 cleavage) or Actin-dNGLUC (control plasmid) were co-transfected with CMV-Luc2 into atg4b−/− MEF cells stably expressing GFP control, or ATG4B WT or various mutants (as labeled). After 48 h, the activity of Gaussia luciferase in the culture medium was measured, and normalized to Firefly luciferase activity in the cells, setting the activity for ATG4B WT as 100%. Error bars indicate the S.D. of three independent experiments. *, p < 0.05; **, p < 0.01. Simultaneously, aliquots of these protein samples were analyzed by immunoblotting using anti-ATG4B and anti-Actin antibodies.