FIGURE 3.
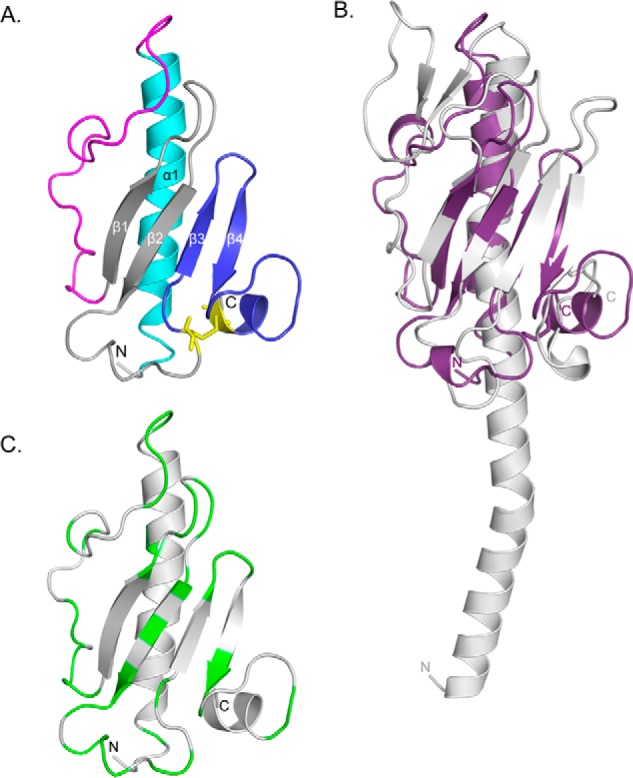
X-ray crystal structure of PilEΔ1–28. A, the x-ray crystal structure of selenomethionine-labeled PilEΔ1–28 was solved to 1.25 Å (PDB code: 4NOA). The N-terminal α-helix is colored cyan, αβ-loop is in magenta, β-sheet is in gray, and D-region is in blue. Cys residues are represented as sticks and colored yellow. B, structural alignment between PilEΔ1–28 (purple) and PilAPAK (gray, PDB code: 1OQW). 96 residues aligned with an RMSD of 3.8 Å. The arrow indicates the hook-like protrusion in both structures. C, mapping of residues differing between PilEPAO1 and PilEPA14. Non-conservative residues are colored green. Structural illustrations and alignments were generated with PyMOL (version 1.3, Schrödinger, LLC).
