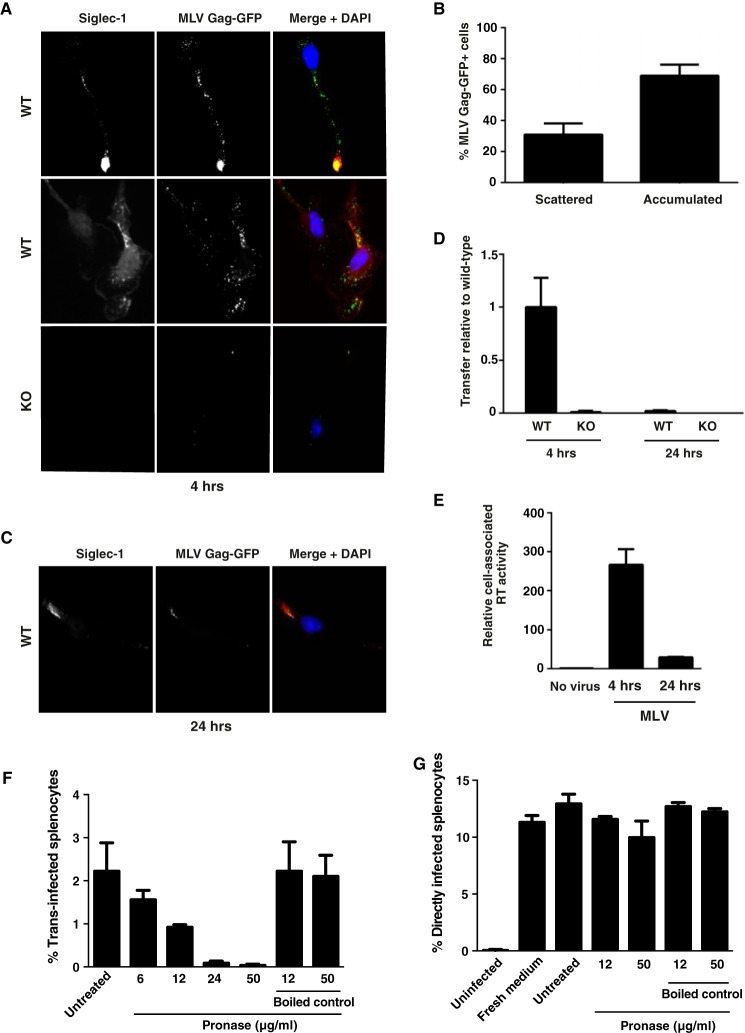
FIGURE 4.
Captured MLV particles partially co-localize with Siglec-1 in BMDM early after virus exposure, and surface-bound MLV is the primary source for trans-infection. BMDM derived from either WT or KO mice were pulsed with MLV Gag-GFP, which carries a Gag-GFP fusion protein (A–C), or MLV-GFP, for 4 h at 37 °C (D and E). PBS-washed BMDM were either co-cultivated with S1A.TB-cells added either immediately after washing (4 h) or 20 h later (24 h) (D) or fixed, permeabilized, and stained using an Alexa647-conjugated anti-Siglec-1 mAb (A–C). Representative images for a dominant single accumulated MLV Gag/Siglec-1 signal (A, upper panels, 4 h) or multiple scattered punctae from WT-BMDM (A, middle panels, 4 h) are shown. A, bottom panel, images of MLV-Gag-GFP-pulsed KO-BMDM stained for mSiglec-1. B, MLV Gag pattern (“scattered” or “accumulated”) and frequency of WT-BMDM displaying this phenotype were quantified. At least 70 cells from each of three mice were analyzed. E, cell-associated RT activity was quantified using SG-PERT, and values are depicted as activity associated with WT cells relative to KO cells. F and G, BMDM were pulsed with MLV-GFP for 4 h at 37 °C. PBS-washed BMDM were treated with increasing concentrations of cleavage-competent active Pronase for 30 min at 4 °C. After Pronase inactivation through washes in FBS-containing medium, cells were co-cultured with LPS-activated splenocytes for assessment of trans-infection. F, BMDM, in the absence of MLV, were treated as in G, then washed with PBS, and cultivated for 1 h more at 37 °C. These culture supernatants were then mixed with MLV-GFP to assess their potential impact on direct MLV-GFP infection of splenocytes.