FIGURE 5.
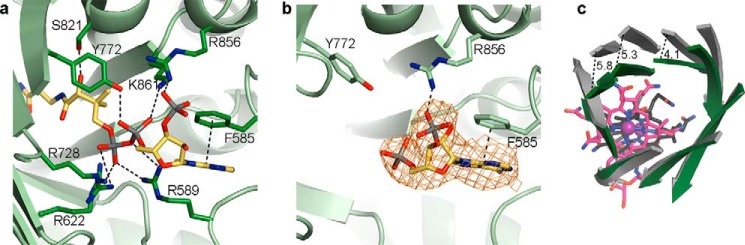
Differences of substrate-binding domains of IcmF dimer. a, isobutyryl-CoA binding to IcmF chain A of the IcmF dimer, which is in a catalytically active conformation. Coloring as in Fig. 4. b, isobutyryl-CoA binding to IcmF chain B, which is in a catalytically inactive conformation. IcmF (green carbons), isobutyryl-CoA (yellow carbons), and 2Fo − Fc electron density (orange mesh), contoured at 1.0 σ, are shown as in Fig. 4a. Note that there is no electron density past the 5′-phosphate of the isobutyryl-CoA nucleotide moiety; therefore, additional atoms were not modeled. The nucleotide portion is bound by few specific interactions, as indicated by black dashed lines. Other interactions between IcmF and isobutyryl-CoA are disrupted because of the conformational change in IcmF chain B compared with IcmF chain A. c, different conformations of substrate-binding domains of IcmF chains A (dark green) and B (gray) isobutyryl-CoA-bound IcmF. Chains are superposed by TIM barrel β-strands. Cbl of chain A is shown as in Fig. 4a, Cbl of chain B is shown with carbons in black. Distances between corresponding Cα atoms are indicated in Å.