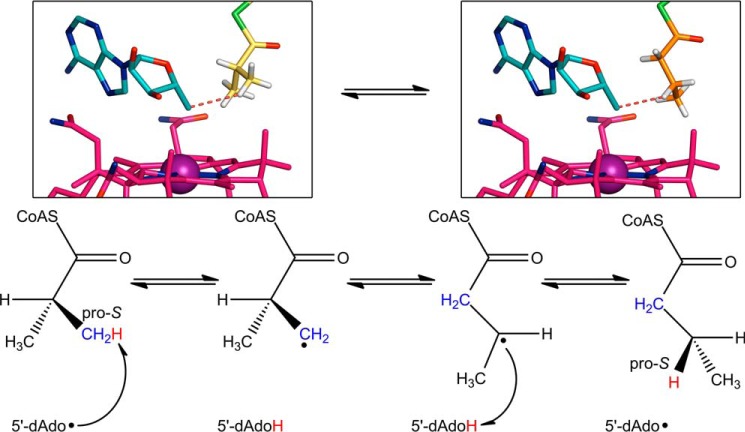
FIGURE 8.
Stereochemical course of isobutyryl-CoA mutase reaction. The chemical mechanism shown at the bottom was established based on stereochemical studies (57). Following Co–C bond homolysis (step not shown), the 5′-dAdo radical abstracts a hydrogen atom (red) from the pro-S methyl group of isobutyryl-CoA (blue). The isobutyryl-CoA radical rearranges to the n-butyryl-CoA radical, which then re-abstracts the hydrogen atom from 5′-deoxyadenosine. The hydrogen atom ends up in the pro-S position. In the reverse reaction, the 5′-dAdo radical abstracts the pro-S hydrogen from n-butyryl-CoA. The structures of IcmF bound to isobutyryl-CoA (left) and n-butyryl-CoA (right) support the proposed stereochemistry. Isobutyryl-CoA (yellow carbons) positions its pro-S methyl group next to the 5′-dAdo group (cyan carbons), whereas n-butyryl-CoA positions its pro-S hydrogen (white sticks) toward the 5′-dAdo group. The red dashed line connects the 5′-dAdo C5′ to the closest hydrogen atom. Hydrogens are modeled based on ideal geometry. Cobalamin is shown with pink carbons and cobalt as a purple sphere.