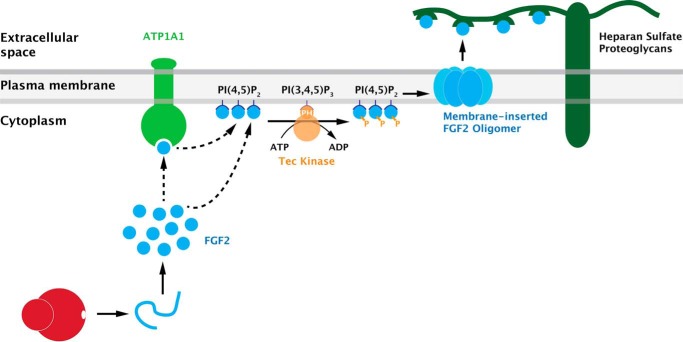
FIGURE 1.
A current view of the molecular mechanism of unconventional secretion of FGF2. FGF2 secretion is mediated by direct translocation across the plasma membrane. This process involves sequential interactions of FGF2 with components at the inner leaflet, including ATP1A1 (the α subunit of the Na/K ATPase), the phosphoinositide PI(4,5)P2, and Tec kinase. As a result, membrane-inserted oligomers form in a PI(4,5)P2-dependent manner, structures that have been interpreted as intermediates in FGF2 membrane translocation. Membrane-proximal heparan sulfate proteoglycans are required to trap FGF2 on cell surfaces, the final step in the overall process of FGF2 secretion. Modified from research originally published in the Journal of Biological Chemistry. Zacherl, S., La Venuta, G., Müller, H. M., Wegehingel, S., Dimou, E., Sehr, P., Lewis, J. D., Erfle, H., Pepperkok, R., and Nickel, W. A direct role for ATP1A1 in unconventional secretion of fibroblast growth factor 2. J. Biol. Chem. 2015; 290: 3654–3665. © the American Society for Biochemistry and Molecular Biology.