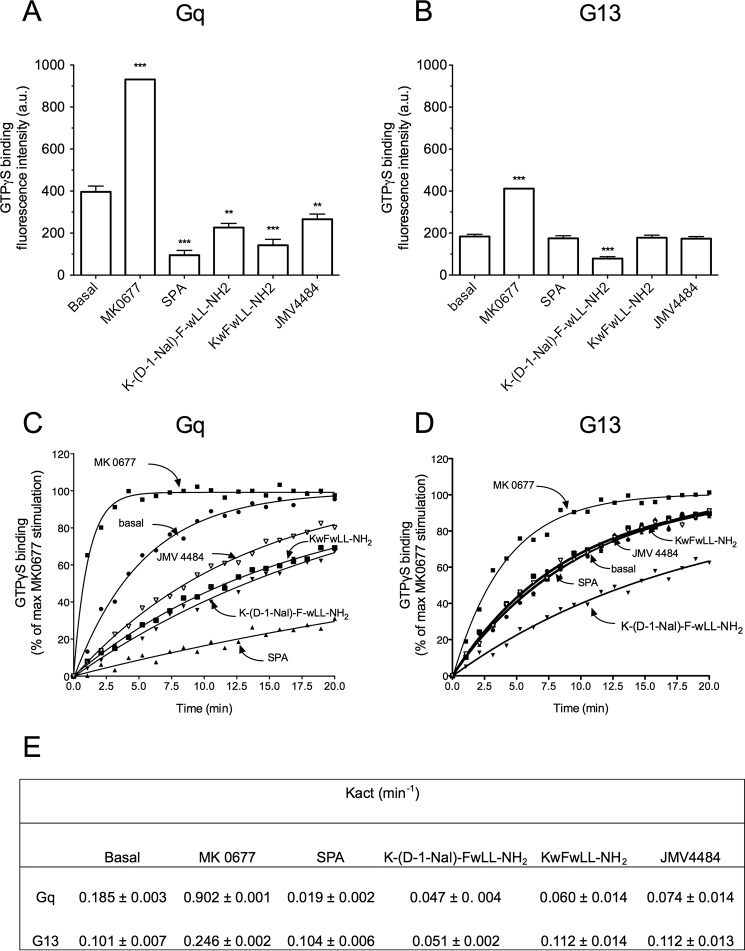
FIGURE 10.
GHS-R1a-dependent constitutive activity at Gq and G13 proteins and ligand selectivity with the purified receptor. The monomeric GHS-R1a in lipid discs was incubated with purified Gαq and Gα13 in the presence of Gβ1γ2. The efficacy of ligands to modulate GHS-R1a-promoted Gq (A) and G13 (B) activity was assessed by monitoring changes in the BODIPY® FL GTPγS emission intensity. GTPγS binding is expressed as raw values of fluorescence emission of BODIPY® FL GTPγS. Data are from one representative of three independent experiments, and statistical significance between unliganded and liganded GHS-R1a was assessed using Student's t test (***, p < 0.001; **, p < 0.01). Kinetics of GTPγS binding to Gq (C) and G13 (D) were carried out under the same conditions. GHS-R1a-catalyzed GTPγS binding is expressed as the percentage of maximal MK-0677 stimulation. E, Kact (min−1) values (mean ± S.E., n = 2) were calculated from GTPγS binding kinetics using GraphPad Prism software.