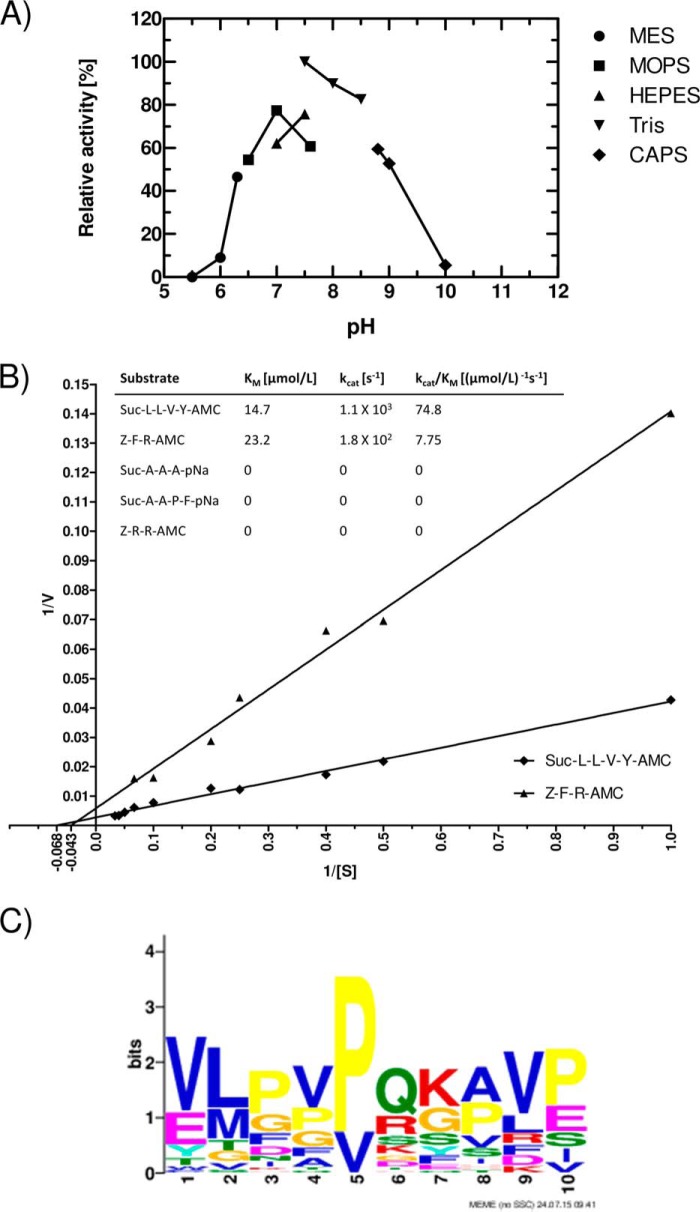
FIGURE 4.
Tpr hydrolyzes Suc-Leu-Leu-Val-Tyr-AMC at a pH in the range from 6.5 to 9.0 (A), obeying Michaelis-Menten kinetics (B), and the enzyme has a broad specificity (C). The pH profile was determined by assaying the activity in buffers of different pH (all containing 5 mm CaCl2 and 1 mm DTT), with substrate added after 30 min of preincubation. Activity (relative fluorescence units/min at the linear phase of fluorescence increase) at 50 mm Tris-HCl, 5 mm CaCl2, and 1 mm DTT, pH 7.5, was arbitrarily taken as 100% (A). Km values for Suc-Leu-Leu-Val-Tyr-AMC and Z-Phe-Arg-AMC were determined from the initial velocity of hydrolysis of substrates at concentrations in the range from 0.5 μm to 30 μm (B). A Tpr cleavage consensus sequence motif was derived from digestion of bovine casein and human fibrinogen by Tpr33 (C). Residues at positions 6 and 7 are equivalent to P1-P1′. The motif was discovered using MEME (Multiple Em for Motif Elicitation) with default parameters except for site distribution = 0 or 1 set for the presence or absence, respectively, of a given residue.