Abstract
Background
The same electrocardiographic (ECG) criteria that have been used for detection of left ventricular hypertrophy (LVH) have recently been recognized as predictors of adverse clinical outcomes, but this predictive ability is inadequately explored and understood.
Methods
A total of 14,984 participants from the Atherosclerosis Risk in Communities (ARIC) study were included in this analysis. Romhilt-Estes (R-E) LVH score was measured from the automatically processed baseline (1987-1989) ECG data. All-cause mortality was ascertained up to December 2010. Cox proportional hazard models were used to examine the association between baseline R-E score, overall and each of its six individual components separately, with all-cause mortality. The associations between change in R-E score between baseline and first follow up visit with mortality was also examined.
Results
During a median follow up of 21.7 years, 4549 all-cause mortality events occurred during follow up. In multivariable adjusted models, increasing levels of the R-E score was associated with increasing risk of mortality both as a baseline finding and as a change between the baseline and the first follow-up visit. Four of the six ECG components of the score were predictive of all-cause mortality [P-terminal force, QRS amplitude, LV strain, and intrinsicoid deflection], while two of the components were not [left axis deviation and prolonged QRS duration]. Differences in the strengths of the associations between the individual components of the score and mortality were observed.
Conclusions
The R-E score, traditionally used for detection of LVH, could be used as a useful tool for predication of adverse outcomes.
Keywords: All-cause mortality, Cardiovascular mortality, Risk assessment, Cardiovascular risk, electrocardiographic risk factors, risk assessment tool, Romhilt-Estes LVH score
Introduction
For most of the past half century, most research in the clinical use of the electrocardiogram (ECG) has been focused on finding a better method for detecting left ventricular hypertrophy (LVH). This search has not been very productive, and better imaging techniques, such as echocardiographic and MRI images, now provide a more precise and accurate assessment of LVH.
The Romhilt-Estes (R-E) Score (1) was one of the early efforts to improve the ability of the ECG to detect increased left ventricular (LV) mass, and was developed before any imaging technologies other than radiography were available. It was based on earlier studies in which ECG tracings of autopsied patients and hemodynamic studies were analyzed for the presence or absence of ECG features previously proposed as indicators of increased LV mass (2, 3). The more “reliable” features as validated by these studies were then used in a point score system, proposed for the ECG diagnosis of LVH.
The R-E score assigned points for the presence of each of six ECG features. If a given ECG reached a total of 5 points, it was considered positive for LVH, and 4 points were considered as probable LVH. The R-E Score proved to be more specific in predicting LV mass than previous systems, but the sensitivity was low, in the range of 60% in the original series of autopsied study patients (1). Similar to all other ECG LVH criteria, attempts to improve sensitivity of R-E score proved fruitless, as each such modification led to an unacceptable increase in false positives. The advent of, and widely increased availability of imaging technology has made optimizing current ECG LVH criteria less relevant.
This study aimed at the quantitation and better understanding of the prognostic significance of the ECG features of the R-E Score as a predictor of all-cause mortality.
Methods
The population used for this analysis included 15,792 participants, aged 45 to 64 years who participated in the Atherosclerosis Risk in Communities (ARIC) Study. This cohort was recruited and first examined in 1987-1989 from 4 US communities. The ARIC study and its methods have been described elsewhere (4). Follow-up visits were carried out in 1990-1992 (93% return rate), 1993-1995 (86%), 1996-1998 (80%) and 2011-2013 (65%).
For the purpose of this analysis, we excluded 808 participants: 196 had no ECG, 136 had ECGs of inadequate quality, 429 had an external pacemaker, Wolff-Parkinson-White pattern or complete bundle branch blocks, and 47 were neither African-American nor white in ethnic origin. No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Electrocardiography
At each study exam, a standard supine 12-lead resting ECG was recorded with a MAC PC Personal Cardiograph [Marquette Electronics, Milwaukee, Wisconsin, USA] and transmitted to the ARIC ECG Reading Center [EPICARE Center, Wake Forest School of Medicine, Winston Salem, NC] for automatic coding. ECGs were automatically processed using Marquette 12-SL Version 2001 [GE, Milwaukee, Wisconsin, USA]. R-E score was calculated from 6 ECG features with a specific value of points for each feature as follows: R or S wave in any limb lead ≥2 mv, or S wave in V1 or V2≥3 mv., or R wave in V5 or V6 ≥3 mv [3 points]; P terminal force defined as terminal negativity of P wave in V1≥ 0.10 mV in depth and ≥ 0.04 msec in duration [3 points]; left ventricular strain defined as ST segment and T wave in opposite direction to QRS in V5 or V6, without digitalis [3 points]; left axis deviation defined as QRS axis ≤ −30 degrees [2 points]; QRS duration ≥0.09 msec [1 point]; and intrinsicoid deflection in V5 or V6 ≥ 0.05 msec [1 point].
Covariates
Baseline age, sex, race, education level, income and smoking status were determined by self-report. Body mass index [BMI] at baseline was calculated as weight [in kilograms] divided by height [in meters] squared. Blood samples were obtained after an 8-hour fasting period. Diabetes was defined as a fasting glucose level ≥126 mg/dL [or non-fasting glucose ≥200 mg/dL], a self-reported physician diagnosis of diabetes, or use of diabetes medications. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of blood pressure lowering medications. Prevalent CVD was identified by self-reported history or a previous physician diagnosis.
Statistical analysis
Baseline R-E scores were calculated for all participants and various baseline characteristics of the population were tabulated and compared across increasing levels of the R-E score, grouped as follows: score=0, 1-3, 4, and >=5. Incidence rates of all-cause mortality per 1000 person-years in each of the R-E score levels occurred during follow up (from visit 2 until December 2010) were calculated, and Kaplan-Meier survival curves were plotted to compare event-free survival across these ascending score levels.
Cox proportional hazards analysis was used to examine the association between R-E score and all-cause mortality in a series of models as follows: Model 1, unadjusted; Model 2, adjusted for age, sex, and race; and Model 3. adjusted for the model 2 variables plus: field center, BMI, systolic blood pressure, smoking status, education, hypertension, diabetes mellitus, cardiovascular disease status, family history of CHD, ratio of total cholesterol/high-density lipoprotein, blood glucose, and serum creatinine at baseline. In these models, R-E score 0 was the reference group and risk of mortality was evaluated in 3 groups of R-E score (1-3, 4, and >=5).
Using similar models, the association between change in the score between the baseline visit and the first return visit with mortality was also examined. The group that exhibited no change served as the reference group for this analysis.
The risk of mortality was also calculated for each of the six components of the score: P-terminal force in V1, QRS voltage, left axis deviation, QRS duration, intrinsicoid deflection time, and ST/T abnormalities (left ventricular strain). Each of these components was evaluated separately as present/absent at the baseline visit, with the absent value group as the reference group. Models were adjusted in a similar fashion as mentioned above but with an additional model 4 in which the 6 components were added to those in model 3.
Statistical significance for all analyses was p<0.05. Analyses were conducted using SAS 9.2 [SAS Institute, Cary, NC].
Results
A total of 14,984 participants [age 54.1± 5.8 years; 55.8 % females; 26.9% African Americans] were included in this analysis. The baseline prevalence of R-E score was as follows: R-E= 0 in 6342 participants, 1-3 in 8017 participants, 4 in 416 participants and 5 or more in 209 participants. Table I shows the participants characteristics across levels of R-E score. Participant characteristics found to be associated with increasing levels of R-E score were age, body mass index, systolic blood pressure, African-American ethnicity, male sex, education level, smoking, diabetes, total cholesterol, hypertension, use of blood-pressure lowering drugs, and history of coronary heart disease. On the other hand, family history of coronary heart disease and statin use did not differ across R-E levels.
Table I.
Baseline (1987-1989) characteristics stratified by levels of Romhilt-Estes score
| Mean (SD) or % | Score =0 n=6342 |
Score ≤3 n=8017 |
Score =4 n=416 |
Score ≥5 n=209 |
P value* |
|---|---|---|---|---|---|
| Age (years) | 54 (5.7) | 54 (5.7) | 56 (5.7) | 56 (5.5) | <.0001 |
| Body mass index (kg/m2) | 27 (5.5) | 28 (5.3) | 28 (5.4) | 28 (5.0) | <.0001 |
| Systolic blood pressure (mmHg) | 120 (18.4) | 121 (18.0) | 134 (24.3) | 137 (29.0) | <.0001 |
| Total cholesterol (mg/dL) | 216 (42.3) | 214 (41.6) | 218 (42.6) | 213 (49.5) | 0.003 |
| Women (%) | 74.4 | 42.5 | 38.7 | 35.4 | <.0001 |
| African-American (%) | 29.1 | 23.4 | 49.0 | 49.8 | <.0001 |
| Education (≤ high school) (%) | 56.9 | 54.8 | 63.2 | 66.0 | <.0001 |
| Smoke (current) (%) | 26.9 | 24.8 | 34.0 | 37.3 | <.0001 |
| Diabetes (%) | 10.9 | 11.7 | 20.9 | 24.5 | <.0001 |
| Hypertension (%) | 30.7 | 35.7 | 59.4 | 70.7 | <.0001 |
| Use of blood pressure lowering drugs (%) | 26.5 | 31.2 | 53.9 | 64.1 | <.0001 |
| Statin use (%) | 0.5 | 0.6 | 1.2 | 1.0 | 0.280 |
| History of coronary heart disease (%) | 2.2 | 4.8 | 18.5 | 35.6 | <.0001 |
| Family history of coronary heart disease (%) | 39.4 | 39.7 | 36.8 | 40.7 | 0.669 |
Statistical significant for categorical variables tested using the chi-square method and for continuous variables the Kruskal-Wallis was used.
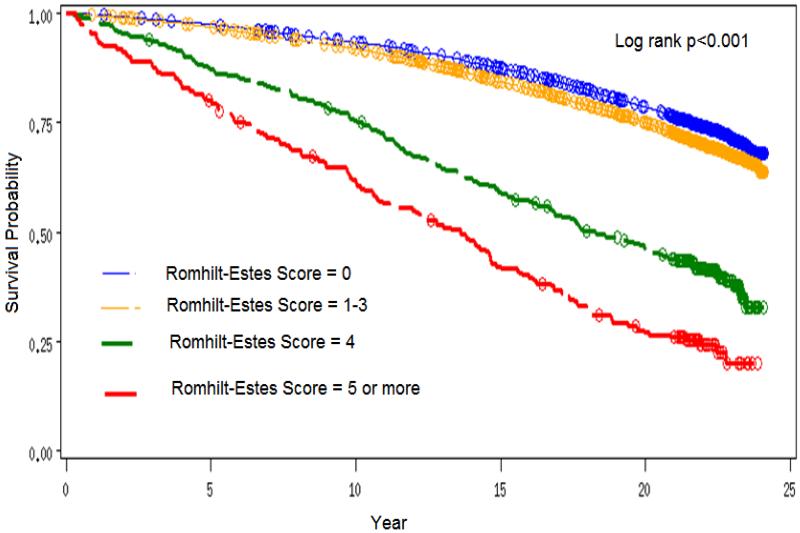
During a median follow up of 21.7 years, 4549 all-cause mortality events occurred. The incidence rate of all-cause mortality was lowest in those with R-E score = 0 and highest in those with R-E score ≥5 [Incidence rates per 1000 person years= 13.8, 16.2, 38.8, and 60.5 in participants with R-E score= 0, 1-3, 4, and ≥5, respectively]. Figure 1 shows the Kaplan Meier survival curves by levels of R-E score.
Figure 1.
Kaplan Meier Survival curves by levels of Romhilt-Estes Score
The risk of all-cause mortality was increasing as the levels of the R-E score increased reaching over four times in those with R-E score ≥ 5 compared to those with R-E score= 0. This pattern of associations remained significant even after adjustment for participant characteristics (Table II).
Table-II.
Baseline (1987-1989) Romhilt-Estes score and risk for all-cause mortality during follow up (up to 2010)
| N | Event rate/ 1000 person years |
Model-1 | p-value | Model-2 | p-value | Model-3 | p-value | |
|---|---|---|---|---|---|---|---|---|
| Score =0 | 6342 | 13.8 | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Score 1-3 | 8017 | 16.2 | 1.18 (1.11-1.26) | <.0001 | 1.05 (0.99-1.12) | 0.126 | 1.00 (0.93-1.07) | 0.937 |
| Score =4 | 416 | 38.8 | 2.67 (2.34-3.05) | <.0001 | 2.06 (1.80-2.36) | <.0001 | 1.60 (1.39-1.84) | <.0001 |
| Score ≥5 | 209 | 60.5 | 4.50 (3.82-5.31) | <.0001 | 3.50 (2.96-4.14) | <.0001 | 2.08 (1.75-2.48) | <.0001 |
Model-1: Unadjusted
Model-2: Adjusted for age, sex and race;
Model-3: Adjusted for demographic and clinical variables of age, sex, race, field center, body mass index, systolic blood pressure, smoking status, education, hypertension, diabetes mellitus, cardiovascular disease status, family history of CHD, ratio of total cholesterol/high-density lipoprotein, blood glucose, and serum creatinine at baseline
Table III shows the risk of mortality associated with each of the six individual components of the R-E score. As shown, four of the six ECG components of the score [P-terminal force in V1, QRS amplitude, LV strain, and intrinsicoid deflection] were predictive of all-cause mortality in the fully adjusted models (which also included all the six components together) while two of the components were not [left axis deviation and prolonged QRS duration]. Differences in the strengths of the associations between the individual components of the score and mortality were also observed.
Table III.
Baseline (1987-1989) components of Romhilt/Estes score and risk for all-cause mortality during follow up (up to 2010)
| Event rate/1000 per- son years |
Model | HR (95%CI) | P-value | ||
|---|---|---|---|---|---|
| Absent | Present | ||||
|
|
|||||
| R or S wave in any limb lead ≥2 mv, or S wave in V1 or V2≥3 mv, or R wave in V5 or V6 ≥3 mv. (n=236) [present vs. absent] |
15.8 | 37.1 | Model 1a | 2.48 (2.09-2.94) | <.0001 |
| Model 2b | 1.81 (1.52-2.15) | <.0001 | |||
| Model 3c | 1.43 (1.19-1.71) | 0.0001 | |||
| Model 4d | 1.21 (1.01-1.46) | 0.0436 | |||
|
| |||||
| Left atrial enlargement: terminal negativi- ty of P wave in V1 ≥ 0.10mV in depth and ≥ 0.04 msec in duration (n=193) [present vs. absent] |
15.8 | 45.2 | Model 1a | 2.60 (2.17-3.10) | <.0001 |
| Model 2b | 2.35 (1.97-2.81) | <.0001 | |||
| Model 3c | 1.74 (1.45-2.09) | <.0001 | |||
| Model 4d | 1.62 (1.34-1.95) | <.0001 | |||
|
| |||||
| Left ventricular strain: ST segment and T wave in opposite direction to QRS in V5 or V6, without digitalis (n=529) [present vs. absent] |
15.2 | 46.4 | Model 1a | 2.90 (2.60-3.23) | <.0001 |
| Model 2b | 2.64 (2.36-2.95) | <.0001 | |||
| Model 3c | 1.83 (1.63-2.06) | <.0001 | |||
| Model 4d | 1.72 (1.53-1.94) | <.0001 | |||
|
| |||||
| Left axis deviation: ≤ (−30) degrees (n=593) [present vs. absent] |
15.7 | 26.5 | Model 1a | 1.49 (1.32-1.69) | <.0001 |
| Model 2b | 1.34 (1.18-1.52) | <.0001 | |||
| Model 3c | 1.14 (1.01-1.30) | 0.0373 | |||
| Model 4d | 1.09 (0.96-1.23) | 0.2075 | |||
|
| |||||
| QRS duration ≥0.09 msec.(n=8194) [present vs. absent] |
14.7 | 17.2 | Model 1a | 1.19 (1.12-1.26) | <.0001 |
| Model 2b | 1.04 (0.98-1.11) | 0.2161 | |||
| Model 3c | 1.00 (0.94-1.06) | 0.9670 | |||
| Model 4d | 0.97 (0.91-1.03) | 0.2846 | |||
|
| |||||
| Intrinsicoid deflection in V5 or V6 ≥ 0.05 msec. (n=504) [present vs. absent] |
15.8 | 25.1 | Model 1a | 1.75 (1.52-2.00) | <.0001 |
| Model 2b | 1.67 (1.45-1.92) | <.0001 | |||
| Model 3c | 1.44 (1.25-1.66) | <.0001 | |||
| Model 4d | 1.38 (1.20-1.60) | <.0001 | |||
Model-1: Unadjusted
Model-2: Adjusted for age, sex and race;
Model-3: Adjusted for demographic and clinical variables of age, sex, race, field center, body mass index, systolic blood pressure, smoking status, education, hypertension, diabetes mellitus, cardiovascular disease status, family history of CHD, ratio of total cholesterol/high-density lipoprotein, blood glucose, and serum creatinine at baseline
Model-4: Adjusted for all demographic and clinical variables in Model 3 plus (instead of and) the total of all six components.
In an attempt to duplicate the clinical situation of a patient being followed by his/her clinician and who develops a higher score between visits, Table IV is presented. This table presents the risk for all-cause mortality associated with a change in R-E score between the baseline and first follow up visit, using the “no change” group as the reference. The risk is determined over the course of followup, to 2010. As seen, there is a steady rise in event rate with each point of increase in the score.
Table IV.
Change in Romhilt/Estes score between baseline (1987-1989) and first follow up visit (1990-1992) and risk for all-cause mortality during follow up (up to 2010)
| N | Event rate (%) |
Model-1 | p-value | Model-2 | p-value | Model-3 | p-value | |
|---|---|---|---|---|---|---|---|---|
| change =0 | 4625 | 22.8 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| change =1 | 5775 | 27.6 | 1.22 (1.11-1.30) | <.0001 | 1.19 (1.10-1.28) | <.0001 | 1.19 (1.10-1.28) | <.0001 |
| change =2 | 2298 | 30.8 | 1.35 (1.22-1.48) | <.0001 | 1.31 (1.19-1.44) | <.0001 | 1.26 (1.14-1.39) | <.0001 |
| change =3 | 714 | 46.1 | 2.23 (1.97-2.52) | <.0001 | 2.13 (1.88-2.41) | <.0001 | 1.74 (1.53-1.98) | <.0001 |
| change ≥4 | 188 | 53.7 | 2.58 (2.11-3.17) | <.0001 | 2.53 (2.06-3.11) | <.0001 | 2.12 (1.71-2.61) | <.0001 |
Model-1: Unadjusted
Model-2: Adjusted for age, sex and race;
Model-3: Adjusted for demographic and clinical variables of age, sex, race, field center, body mass index, systolic blood pressure, smoking status, education, hypertension, diabetes mellitus, cardiovascular disease status, family history of CHD, ratio of total cholesterol/high-density lipoprotein, blood glucose, and serum creatinine at baseline
Discussion
Cardiologists of the mid-20th century recognized that clinical signs of LVH were an adverse development, and the ECG was seen as a noninvasive tool for earlier detection of cardiac (usually LV) enlargement, at a time when there was no noninvasive alternative available beyond a chest roentgenogram. Thus the focus of research at that time was in developing more sensitive and precise techniques of obtaining an ECG “diagnosis” of LVH. Today, information about LV mass is now easily provided by imaging techniques, such as echo and MRI, and these techniques are clearly better at this task than the ECG. Some have questioned whether of not the ECG is relevant in routine followup of patients with heart disease, and the U.S. Preventive Services Task Force has advised that it not be used as a screening tool for detection of coronary heart disease in asymptomatic patients (5).
Two facts have emerged over the past several decades which have refocused electrocardiographic research. One is the demonstration that the same ECG changes we once used to “diagnose” an increase in LV mass have the ability to predict an adverse course of the underlying disease, independent of LV mass (6, 7, 8). The other is the demonstration that these changes are reversible with effective therapy, and their disappearance signals a favorable turn in the course of the underlying illness (9,10). If these early observations can be validated, quantitated and expanded, we would likely have evidence adequate to support use of the ECG as an evidence based guide to treatment, indicating a need for change in treatment, and serving as an added incentive to the patient as he/she adapts to tightened therapy or altered lifestyle.
Some groups, such as the Working Group on the Electrocardiographic Diagnosis of Left Ventricular Hypertrophy (11), have urged that the search for a better ECG indicator of increased LV mass be abandoned in favor of research on the role of the ECG as a predictor of increased risk. This group also advocated that the term ECG/LVH be replaced by a more appropriate term, such as electrical remodeling, recognizing that these effects may not be due to increased mass, but to the influence of some yet unknown precursor condition, such as fibrosis, which causes both the electrical effect and increased mass (12,13).
As demonstrated above, the R-E score, as originally proposed for the “diagnosis” of LVH, also predicts an increase in all-cause mortality at a highly significant level, and a further increase in the point score from one visit to the next is equally striking as an indicator of increased risk. The conclusion is that the original R-E score, as such, is a powerful predictive tool for all-cause mortality.
In addition, this analysis shows that the majority of the individual ECG components that comprise the R-E score are independently predictive of all-cause mortality. Specifically, the P-terminal force, ST-T changes of left ventricular strain, and the duration of the “intrinsicoid deflection” are all strong predictors of all-cause mortality. Interestingly, QRS amplitude, the component given highest value in most ECG-LVH criteria, is the least powerful component of the set.
Each of the components of the R-E score represents a different variation in electrical events within the myocardium, but we have little information about the precise alterations that underlie these ECG “patterns”. It is possible that each of the four predicative components signals a different electrical event within the myocardium, and a different ability to predict specific cardiovascular outcomes. It is also likely that other ECG patterns will prove to have the same ability to predict adverse cardiovascular events, and could join the above set of four. These and other questions are subjects for future investigations.
The results of this study clearly imply potential usefulness of the ECG as a predictive tool in clinical care of patients with cardiovascular disease. The set identified in this study are those generated by autopsy and hemodynamic studies almost a half century ago for another purpose. It seems likely that they can be refined and clarified by further study, and made even more powerful. More specifically, the independent ability of the four risk predictive components of the score must be evaluated for the ability of each to predict specific forms of cardiovascular disease. These findings make the objective of a validated, non-invasive clinical tool a more likely possibility, and worthy of further study.
Limitations
The population studied was restricted to those with white and African-American ethnicity. Other ethnic groups must be studied. The ECG findings in this study were from ECGs recorded by specially trained technicians, using more sensitive recording devices than those in the usual hospital or outpatient setting. The effect of less rigorous electrode placement and lower frequency response of recording instruments are unknown. This study only examines the risk of all-cause mortality. The risk of cardiovascular mortality and the possible differential effects of the R-E score components on different types of cardiovascular illness or mortality will be explored as the next step.
Conclusions
The R-E score is highly predictive of all-cause mortality, both as a single baseline score, and as an increasing score over time. The six individual ECG components of theR-E score contain four components with independent predictive ability. Each deserves further study of its differential predictive ability for different cardiovascular events. The study results strengthen the likelihood that a set of ECG parameters can be identified and validated as a risk assessment tool, useful for guidance of the physician caring for a patient with cardiovascular disease.
Highlights.
-
*
The Romhilt-Estes Score, used for decades as an indicator of the presence of LVH also has the independent ability to predict all cause mortality.
-
*
Four of the six components of the Score have the ability to predict increased mortality, each independent of the others.
-
*
Of the four components with predictive ability, QRS amplitude has the least predictive strength.
-
*
The strength of these relationships indicates that they may have value as a predictive tool for the clinician caring for patients with cardiovascular diseases.
Acknowledgements
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contribution.
Footnotes
Disclosures:
Dr. Estes has filed a provisional application for a patent related to automated generation of a risk score from an ECG, delivering the score to the physician as a part of the machine generated report.
References
- 1.Romhilt DW, Estes EH. A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J. 1968;75:752–8. doi: 10.1016/0002-8703(68)90035-5. [DOI] [PubMed] [Google Scholar]
- 2.Carter WA, Estes EH. Electrocardiographic manifestations of ventricular hypertrophy; a computer study of ECG-anatomic correlations in 319 cases. Am Heart J. 1964;68:173–82. doi: 10.1016/0002-8703(64)90038-9. [DOI] [PubMed] [Google Scholar]
- 3.Morris JJ, Estes EH, Whalen RE, et al. P-wave analysis in valvular heart disease. Circulation. 1964;29:242–252. doi: 10.1161/01.cir.29.2.242. [DOI] [PubMed] [Google Scholar]
- 4.The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989 Apr;129(4):687–702. [PubMed] [Google Scholar]
- 5.Moyer VA, U.S. Preventive Services Task Force Screening for coronary heart disease with electrocardiography: U.S. Preventive Services Task Force Recommendation Statement. Ann. Int. Med. 2012;157:512–19. doi: 10.7326/0003-4819-157-7-201210020-00514. [DOI] [PubMed] [Google Scholar]
- 6.Sundstrom J, Lind L, Arnlo J, et al. Echocardiographic and electrocardiographic diagnosis of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103:2346–51. doi: 10.1161/01.cir.103.19.2346. [DOI] [PubMed] [Google Scholar]
- 7.Havranek EP, Froshaug DB, Emserman CD, et al. Left ventricular hypertrophy and cardiovascular mortality by race and ethnicity. Am J Med. 2008;121:870–5. doi: 10.1016/j.amjmed.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rautaharju PM, Soliman EZ. Electrocardiographic left ventricular hypertrophy and the risk of adverse cardiovascular events: A critical appraisal. J Electrocardiol. 2014;47:649–54. doi: 10.1016/j.jelectrocard.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Kurisu S, Inoui I, Kawagoe T, et al. The decrease in QRS amplitude after aortic valve replacement in patients with aortic valve stenosis. J. Electrocardiol. 2009;42:410–3. doi: 10.1016/j.jelectrocard.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Okin PM, Devereux RB, Liu JE, et al. Regression of electrocardiographic left ventricular hypertrophy predicts regression of echocardiographic left ventricular mass: the LIFE study. J. Hum Hypertension. 2004;18:403–9. doi: 10.1038/sj.jhh.1001707. [DOI] [PubMed] [Google Scholar]
- 11.Bacharova L, Working Group on Electrocardiographic Diagnosis of Left Ventricular Hypertrophy The first statement of the Working Group on Electrocardiographic Diagnosis of Left Ventricular Hypertrophy. J. Electrocard. 2010;43:197–9. doi: 10.1016/j.jelectrocard.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Mayosi BM, Avery PJ, Farrall M, et al. Genome wide linkage analysis of electrocardiographic and echocardiographic left ventricular hypertrophy in families with hypertension. Eur Heart J. 2008;29:525–30. doi: 10.1093/eurheartj/ehn028. [DOI] [PubMed] [Google Scholar]
- 13.Bacharova L, Chen H, Estes EH, et al. Determinants in detection and comparison of the prognostic significance of left ventricular hypertrophy by electrocardiogram and cardiac magnetic resonance imaging. Am J Cardiol. 2015;115:515–22. doi: 10.1016/j.amjcard.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]