Abstract
Background:
We evaluated a large-scale transition of primary care physicians to blended capitation models and team-based care in Ontario, Canada, to understand the effect of each type of reform on the management and prevention of chronic disease.
Methods:
We used population-based administrative data to assess monitoring of diabetes mellitus and screening for cervical, breast and colorectal cancer among patients belonging to team-based capitation, non–team-based capitation or enhanced fee-for-service medical homes as of Mar. 31, 2011 (n = 10 675 480). We used Poisson regression models to examine these associations for 2011. We then used a fitted nonlinear model to compare changes in outcomes between 2001 and 2011 by type of medical home.
Results:
In 2011, patients in a team-based capitation setting were more likely than those in an enhanced fee-for-service setting to receive diabetes monitoring (39.7% v. 31.6%, adjusted relative risk [RR] 1.22, 95% confidence interval [CI] 1.18 to 1.25), mammography (76.6% v. 71.5%, adjusted RR 1.06, 95% CI 1.06 to 1.07) and colorectal cancer screening (63.0% v. 60.9%, adjusted RR 1.03, 95% CI 1.02 to 1.04). Over time, patients in medical homes with team-based capitation experienced the greatest improvement in diabetes monitoring (absolute difference in improvement 10.6% [95% CI 7.9% to 13.2%] compared with enhanced fee for service; 6.4% [95% CI 3.8% to 9.1%] compared with non–team-based capitation) and cervical cancer screening (absolute difference in improvement 7.0% [95% CI 5.5% to 8.5%] compared with enhanced fee for service; 5.3% [95% CI 3.8% to 6.8%] compared with non–team-based capitation). For breast and colorectal cancer screening, there were no significant differences in change over time between different types of medical homes.
Interpretation:
The shift to capitation payment and the addition of team-based care in Ontario were associated with moderate improvements in processes related to diabetes care, but the effects on cancer screening were less clear.
Health care systems with a strong primary care orientation have better health outcomes, lower costs and fewer disparities across population subgroups.1 Countries around the world have been experimenting with reforms to improve the delivery of primary care, changing the way physicians are organized and paid. In the United States, several national organizations2,3 and policy experts4,5 have advocated a shift away from fee for service toward capitation or blended payments, and in 2015, the Centers for Medicare and Medicaid Services brought in blended payment in primary care, introducing a non–visit-based payment for chronic care management.6
Patient-centred medical homes have provided an opportunity to transition physicians to new payment models, but they also necessitate changes in care delivery, including incorporation of team-based care, enhancement of access for patients, coordination of care and a focus on quality and safety.7–9 Evidence suggests that team-based care is a particularly important element in improving the management and prevention of chronic disease and reducing related costs.10 Early evaluations of patient-centred medical home pilots were promising,11,12 but a recent study of large-scale implementation showed limited improvements in the quality of chronic disease care and no reduction in health care utilization or total costs over 3 years.13
Before 2002, primary care physicians in Ontario, Canada, were almost universally paid through a fee-for-service system. Over the past decade, more than three-quarters have transitioned to patient-centred medical homes.14,15 About half of Ontario physicians working in patient-centred medical homes have shifted to blended capitation payments, with a portion of these physicians working in groups that also receive government funding for nonphysician health professionals to enable team-based care. However, about 40% of physicians in patient-centred medical homes still receive most of their income through fee-for-service payments. This natural health policy experiment offers a unique opportunity to compare the effectiveness of different payment models and team-based care. Early studies have shown small differences in the quality of cardiovascular16 and diabetes mellitus17 care between physicians receiving capitation payments and those receiving fee-for-service payments, but no studies have assessed changes in quality of care over time.
We evaluated a large-scale transition of primary care physicians to blended capitation models and team-based care to understand the effect of each type of reform on chronic disease management and prevention over time.
Methods
Setting
Ontario is Canada’s largest province, with a population of 13.7 million people in 2014.18 Necessary physician visits, hospital services and medical tests are fully insured for all permanent residents by the Ontario Health Insurance Plan (OHIP) and are free at the point of care, with no copayments or deductibles.
Medical homes were introduced in Ontario between 2002 and 2007.15 Entry into medical homes was voluntary for both primary care physicians and patients, and physicians could select among several types of medical homes. All medical homes included some form of blended physician payment (some capitation and some fee for service), formal patient enrolment, after-hours coverage and financial incentives for preventive health and chronic disease management. Differences between medical homes were based on the amount of capitation payment relative to fee for service and whether the medical home was eligible for funding to hire nonphysician health professionals. In all cases, capitation payments were adjusted for age and sex but not comorbidity. There were no specific incentives to improve care coordination or to engage in performance measurement and improvement (see Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150579/-/DC1).
Study design and patient population
We stratified all Ontario residents (n = 13 161 935) by the type of payment their physician received and whether the physician worked in a team-based setting as of Mar. 31, 2011. We performed a cross-sectional analysis in 2011 to understand the relationship between, on the one hand, physician payment and team-based care and, on the other hand, chronic disease management and prevention, with adjustment for patient and physician characteristics. The outcomes were evidence-based testing for diabetes and recommended screening for cervical, breast and colorectal cancer. We performed a longitudinal analysis to determine whether differences observed in 2011 were pre-existing or could be explained by relative differences in improvement over time. We followed the patients back in time to 2001 and assessed their eligibility for and receipt of recommended testing for each fiscal year until 2011. Physicians and patients transitioned between several types of medical homes at different points in the study period, which precluded a prospective analysis.
We excluded from the outset patients who attended a community health centre (n = 139 274 [1.1%]). For this study, we were unable to capture laboratory tests done in hospitals. To identify physicians who used hospital laboratories, we calculated the average number of outpatient laboratory tests performed per patient over a 1-year period for each study physician and examined the distribution. We identified physician outliers with low rates of outpatient laboratory testing (less than an average of 3 laboratory tests per patient per year) and excluded their patients from our primary analysis (n = 578 942 [4.4%]). We included these patients in a secondary analysis.
We used population-based administrative claims data accessed through a comprehensive research agreement between the Institute for Clinical Evaluative Sciences (ICES) and the Ontario Ministry of Health and Long-Term Care. These data sets were linked using unique, encoded identifiers and were analyzed at ICES. This study was approved by the Research Ethics Board of Sunnybrook Health Sciences Centre in Toronto.
Patient-centred medical homes
We categorized medical homes into 3 categories according to the type of physician payment and whether the medical homes include nonphysician health professionals.
In the enhanced fee-for-service category, physicians receive about 15% of remuneration from capitation, 80% from fee-for-service billings and 5% from incentives and bonuses. This category includes the Family Health Group introduced in 2003 and the Comprehensive Care Model introduced in 2005.
With non–team-based capitation, physicians receive about 70% of remuneration from capitation, 20% from fee-for-service billings and 10% from incentives and bonuses, with no funding for allied health professionals. This category includes the Family Health Network introduced in 2002 and the Family Health Organization introduced in 2007.
In the team-based capitation category, physician payment is the same as under non–team-based capitation, but physicians are eligible for funding to hire allied health professionals. This category represents the Family Health Team introduced in 2005.
We used enrolment tables provided by the Ontario Ministry of Health and Long-Term Care to assign enrolled patients to physicians practising in medical homes as of Mar. 31, 2011. We used virtual rostering for patients who were not formally enrolled in a medical home, assigning them to the physician who billed the maximum value of 18 common primary care fee codes billed within the previous 2 years (Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150579/-/DC1); on this basis, some nonenrolled patients were assigned to physicians practising in medical homes. We assigned both enrolled and nonenrolled patients to a physician panel because we thought this approach best approximated a physician’s true panel of patients, especially for physicians practising in an enhanced fee-for-service model, where there was a lower financial incentive to enrol patients (as only 15% of income was based on capitation payments).
Chronic disease management and prevention
For patients aged 40 years or older identified as having diabetes in the 2 years before Mar. 31 of the fiscal year, we assessed the frequency of retinal eye examination, hemoglobin A1C measurement and cholesterol measurement using physician, optometrist and laboratory service claims. We used a validated algorithm with 86% sensitivity and 97% specificity to identify patients with diabetes.19 Optimal monitoring for a patient with diabetes was defined as 1 retinal eye exam, 4 hemoglobin A1C tests and 1 cholesterol test in a 2-year period, based on recommendations in the Canadian Diabetes Association 2008 clinical practice guidelines.20
We calculated cancer screening rates using service claims. We determined eligibility for and completion of screening on the basis of clinical practice guideline recommendations and the cancer screening bonuses available to medical home physicians, as we have done previously21 (Appendix 3, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150579/-/DC1).
Other data sources
Patient age, sex and place of residence were obtained from the registry of people covered by OHIP. We obtained neighbourhood income quintiles by dissemination area by linking 2006 census data to patients’ residential postal codes. We assessed patients’ rurality of residence using the Rurality Index of Ontario.22 We used citizenship and immigration records to determine whether patients had immigrated to Ontario within the previous 10 years, and we used registration with OHIP within the previous 10 years to identify other residents new to the province. We derived mental health diagnoses from physician billing claims in the previous 2 years using a validated algorithm.23 We based other diagnoses on previously validated cumulative cohorts derived from physician billing claims and hospital discharge abstracts. We used the Johns Hopkins Adjusted Clinical Group software to assess patient comorbidity using adjusted diagnosis groups and to assign patients to resource utilization bands based on similar expected health care utilization (1 = low, 5 = high) over a 2-year period.24 We used physician databases to identify demographic data for physicians practising in Ontario.
Statistical analysis
We used Poisson regression models to examine the association between type of patient-centred medical home and recommended testing for diabetes and cancer screening in 2011. We chose this type of analysis because our outcomes of interest were relatively common, and we wanted to express the effects in terms of relative risks.25 Independent variables were patient age, rurality, income quintile, morbidity (resource utilization band) and immigration status; physician characteristics were age, sex, number of rostered patients and whether the physician was a Canadian medical graduate. We used generalized estimating equations to account for the correlation in outcomes between patients who see the same physician and physicians belonging to the same group.26 Our primary analysis excluded patients of physicians with low rates of outpatient laboratory testing. We performed a secondary analysis that included these patients.
We used a fractional polynomials method developed by Long and Ryoo27 to perform a longitudinal analysis to determine whether patient-level differences observed in 2011 predated the introduction of medical homes in Ontario. We stratified patients by the type of patient-centred medical home to which they belonged in 2011 and followed them back to 2001, assessing the proportion who received diabetes testing and cancer screening in each fiscal year in the study time frame. We defined the cohort in 2011 for each outcome using the eligibility criteria described above. We excluded patients from the denominator for a given year if they were no longer eligible for testing, because of age or diagnosis. For each outcome, we used fractional polynomials to fit a nonlinear model to the observed proportion of patients tested annually over the study time frame. We used the fitted model to compare the change in proportion tested over time between each type of patient-centred medical home.28 Our analysis was based on annual aggregated data that followed the same group of patients back in time, so it was not necessary to adjust for potential confounders. We tested for autocorrelation using the residuals of the model fitted to the annual aggregated data and found no significant autocorrelation.
Results
Patient and physician characteristics by type of medical home
In 2011, medical homes accounted for 10 675 480 Ontarians (81%) and 7095 (84%) of Ontario’s primary care physicians (Table 1, Table 2). Patient and physician characteristics differed by type of medical home. For example, higher proportions of enhanced fee-for-service medical homes were located in urban areas, served new immigrants and patients with higher comorbidity, and included international medical graduates and physicians aged 65 years and older.
Table 1:
Patient characteristics by type of medical home in Ontario, as of Mar. 31, 2011
| Characteristic | Type of medical home; no. (%) of patients* | No. (%) of all patients in Ontario† | ||
|---|---|---|---|---|
| Team-based capitation | Non–team-based capitation | Enhanced fee for service | ||
| No. of patients | 2 412 291 | 3 230 189 | 5 033 000 | 13 161 935 |
| Sex, male | 1 132 812 (47) | 1 535 955 (48) | 2 398 225 (48) | 6 466 459 (49) |
| Age group, yr | ||||
| < 19 | 507 696 (21) | 648 466 (20) | 1 055 402 (21) | 2 913 341 (22) |
| 19–44 | 779 220 (32) | 1 071 406 (33) | 1 836 052 (36) | 4 646 525 (35) |
| 45–64 | 721 279 (30) | 978 505 (30) | 1 454 919 (29) | 3 735 402 (28) |
| ≥ 65 | 404 096 (17) | 531 812 (16) | 686 627 (14) | 1 866 667 (14) |
| Income quintile | ||||
| 1 (low) | 429 008 (18) | 511 905 (16) | 967 804 (19) | 2 485 684 (19) |
| 2 | 463 214 (19) | 578 977 (18) | 1 010 744 (20) | 2 557 363 (19) |
| 3 | 476 822 (20) | 628 572 (19) | 1 051 373 (21) | 2 624 984 (20) |
| 4 | 521 318 (22) | 714 470 (22) | 1 072 344 (21) | 2 773 236 (21) |
| 5 (high) | 511 907 (21) | 786 120 (24) | 917 342 (18) | 2 668 771 (20) |
| Missing data | 10 022 (<1) | 10 145 (<1) | 13 393 (<1) | 51 897 (<1) |
| Immigration status | ||||
| Immigrated in past 10 yr | 55 339 (2) | 122 358 (4) | 526 081 (10) | 920 619 (7) |
| Other newcomer in past 10 yr | 52 078 (2) | 73 578 (2) | 175 392 (3) | 454 419 (3) |
| Long-term resident | 2 304 874 (96) | 3 034 253 (94) | 4 331 527 (86) | 11 786 897 (90) |
| Rurality index | ||||
| Major urban | 1 249 855 (52) | 2 180 293 (68) | 4 269 320 (85) | 9 544 639 (73) |
| Non–major urban | 754 757 (32) | 792 425 (25) | 597 243 (12) | 2 532 267 (19) |
| Rural | 378 431 (16) | 243 442 (8) | 148 767 (3) | 978 832 (7) |
| Missing data | 29 248 (1) | 14 029 (<1) | 17 670 (<1) | 111 197 (1) |
| Resource utilization band,‡ mean ± SD | 2.62 ± 1.13 | 2.67 ± 1.09 | 2.77 ± 1.02 | 2.54 ± 1.20 |
| Adjusted diagnosis groups | ||||
| No utilization | 162 519 (7) | 178 111 (6) | 187 004 (4) | 1 304 747 (10) |
| 1–4 (low comorbidity) | 1 258 840 (52) | 1 575 270 (49) | 2 200 542 (44) | 6 069 330 (46) |
| 5–9 | 834 317 (35) | 1 227 831 (38) | 2 142 350 (43) | 4 773 571 (36) |
| ≥ 10 (high comorbidity) | 156 615 (6) | 248 977 (8) | 503 104 (10) | 1 014 287 (8) |
| Chronic disease | ||||
| Hypertension | 530 704 (22) | 744 882 (23) | 1 104 240 (22) | 2 690 300 (20) |
| Congestive heart failure | 48 487 (2) | 64 281 (2) | 80 528 (2) | 221 121 (2) |
| Acute myocardial infarction | 34 978 (1) | 43 285 (1) | 52 847 (1) | 148 730 (1) |
| COPD | 165 724 (7) | 209 316 (6) | 276 815 (6) | 743 649 (6) |
| Asthma | 328 795 (14) | 465 793 (14) | 762 500 (15) | 1 858 465 (14) |
| Mental illness | 440 002 (18) | 663 158 (21) | 1 129 405 (22) | 2 524 459 (19) |
| Diabetes mellitus and age > 40 yr | 199 265 (8) | 283 550 (9) | 458 513 (9) | 1 065 721 (8) |
Note: COPD = chronic obstructive pulmonary disease, SD = standard deviation.
Except where indicated otherwise. These columns include data for nonenrolled patients assigned (by virtual rostering) to physicians practising in medical homes.
The data in this column represent all Ontario residents, including those not attached (by formal enrolment or virtual rostering) to a medical home; the numbers in this column are therefore greater than the sum of the 3 medical home categories.
For resource utilization bands, 1 = low utilization and 5 = high utilization.
Table 2:
Physician characteristics and panel size by type of medical home in Ontario, as of Mar. 31, 2011
| Characteristic | Type of medical home; no. (%) of physicians* | No. (%) of all comprehensive primary care physicians† | ||
|---|---|---|---|---|
| Team-based capitation | Non–team-based capitation | Enhanced fee for service | ||
| No. of physicians | 1776 | 2051 | 3268 | 8422 |
| Sex, male | 1002 (56) | 1218 (59) | 1956 (60) | 4961 (59) |
| Age group, yr | ||||
| < 40 | 394 (22) | 325 (16) | 460 (14) | 1476 (17) |
| 40–64 | 1252 (71) | 1540 (75) | 2341 (72) | 5874 (70) |
| ≥ 65 | 129 (7) | 186 (9) | 463 (14) | 1064 (13) |
| Missing data | ≤ 5 (<1) | 0 (0) | ≤ 5 (<1) | 8 (<1) |
| Country of medical graduation | ||||
| Canada | 1500 (85) | 1703 (83) | 2081 (64) | 6190 (73) |
| Other country | 275 (15) | 348 (17) | 1183 (36) | 2224 (26) |
| Missing data | ≤ 5 (<1) | 0 (0) | ≤ 5 (<1) | 8 (<1) |
| Panel size, median (IQR) | 1306 (871–1789) | 1496 (1065–2005) | 1398 (944–2008) | 1290 (800–1859) |
| Panel size | ||||
| 0–649 | 243 (14) | 108 (5) | 412 (13) | 1585 (19) |
| 650–999 | 331 (19) | 313 (15) | 499 (15) | 1339 (16) |
| 1000–1499 | 503 (28) | 609 (30) | 880 (27) | 2154 (26) |
| 1500–1999 | 406 (23) | 506 (25) | 644 (20) | 1637 (19) |
| 2000–2399 | 192 (11) | 284 (14) | 335 (10) | 835 (10) |
| ≥ 2400 | 101 (6) | 231 (11) | 498 (15) | 872 (10) |
Note: IQR = interquartile range.
Except where indicated otherwise.
Physicians were considered comprehensive if they worked 50 or more days per year, if 50% or more of their services reflected core primary care, if 50% or fewer of their billings were in a single activity area and if they did not have a “focus practice” designation (e.g., psychotherapy, hospitalist, sports medicine). “All comprehensive primary care physicians” includes those who did not practise in a medical home; therefore, the numbers in this column are greater than the sum of the 3 medical home categories.
Cross-sectional analysis
Patients in team-based capitation practices had the highest rates of recommended testing for diabetes, cervical cancer screening and breast cancer screening (39.7%, 69.2% and 76.6%, respectively), whereas those in enhanced fee-for-service practices had the lowest rates (31.6%, 66.3% and 71.5%, respectively) (Table 3). Rates of screening for colorectal cancer were similar in team-based and non–team-based capitation practices (63.0% and 63.5%, respectively), and these rates were higher than the rate in enhanced fee-for-service practices (60.9%). After adjustment for patient and physician characteristics, the biggest differences were observed for diabetes care, with patients in capitation models more likely to receive recommended testing than those in enhanced fee-for-service practices (team-based capitation, relative risk [RR] 1.22 [95% confidence interval [CI] 1.18 to 1.25]; non–team-based capitation, RR 1.10 [95% CI 1.07 to 1.14]). Patients in capitation models were also more likely than those in enhanced-fee-for-service models to receive breast cancer screening (team-based capitation, RR 1.06 [95% CI 1.06 to 1.07]; non–team-based capitation, 1.04 [95% CI 1.03 to 1.05]) and colorectal cancer screening (team-based capitation, RR 1.03 [95% CI 1.02 to 1.04]; non–team-based capitation, RR 1.04 [95% CI 1.03 to 1.05]); however, there were no differences between models for cervical cancer screening (team-based capitation, RR 1.00 [95% CI 0.99 to 1.01]; non–team-based capitation, RR 1.01 [95% CI 1.00 to 1.02]). Results from the secondary analysis, which included patients of physicians with low rates of outpatient laboratory testing, were similar (results not presented).
Table 3:
Association between enrolment in payment model and chronic disease management and prevention, as of Mar. 31, 2011
| Type of screening and payment model | Crude rate, % | Type of analysis;* RR (95% CI) | ||
|---|---|---|---|---|
| Unadjusted | Adjusted for patient† characteristics | Adjusted for patient† and physician‡ characteristics | ||
| Recommended testing for diabetes | ||||
| Team-based capitation | 39.7 | 1.24 (1.20 to 1.28) | 1.24 (1.20 to 1.28) | 1.22 (1.18 to 1.25) |
| Non–team-based capitation | 36.2 | 1.12 (1.09 to 1.15) | 1.12 (1.09 to 1.15) | 1.10 (1.07 to 1.14) |
| Enhanced fee for service | 31.6 | Reference | Reference | Reference |
| Cervical cancer screening | ||||
| Team-based capitation | 69.2 | 1.07 (1.06 to 1.09) | 0.99 (0.99 to 1.00) | 1.00 (0.99 to 1.01) |
| Non–team-based capitation | 67.5 | 1.05 (1.04 to 1.06) | 1.00 (1.00 to 1.01) | 1.01 (1.00 to 1.02) |
| Enhanced fee for service | 66.3 | Reference | Reference | Reference |
| Breast cancer screening | ||||
| Team-based capitation | 76.6 | 1.10 (1.09 to 1.11) | 1.08 (1.07 to 1.09) | 1.06 (1.06 to 1.07) |
| Non–team-based capitation | 74.5 | 1.07 (1.06 to 1.08) | 1.05 (1.04 to 1.06) | 1.04 (1.03 to 1.05) |
| Enhanced fee for service | 71.5 | Reference | Reference | Reference |
| Colorectal cancer screening | ||||
| Team-based capitation | 63.0 | 1.08 (1.06 to 1.09) | 1.05 (1.03 to 1.06) | 1.03 (1.02 to 1.04) |
| Non–team-based capitation | 63.5 | 1.08 (1.07 to 1.10) | 1.05 (1.04 to 1.07) | 1.04 (1.03 to 1.05) |
| Enhanced fee for service | 60.9 | Reference | Reference | Reference |
Note: CI = confidence interval, RR = relative risk.
Adjustment was performed with Poisson regression models, using generalized estimating equations to account for clustering at the physician level.
Adjustment for the following patient characteristics: age, rurality, income quintile, morbidity (resource utilization band) and immigration status.
Adjustment for the following physician characteristics: age, sex, number of rostered patients and whether a Canadian medical graduate.
Longitudinal analysis
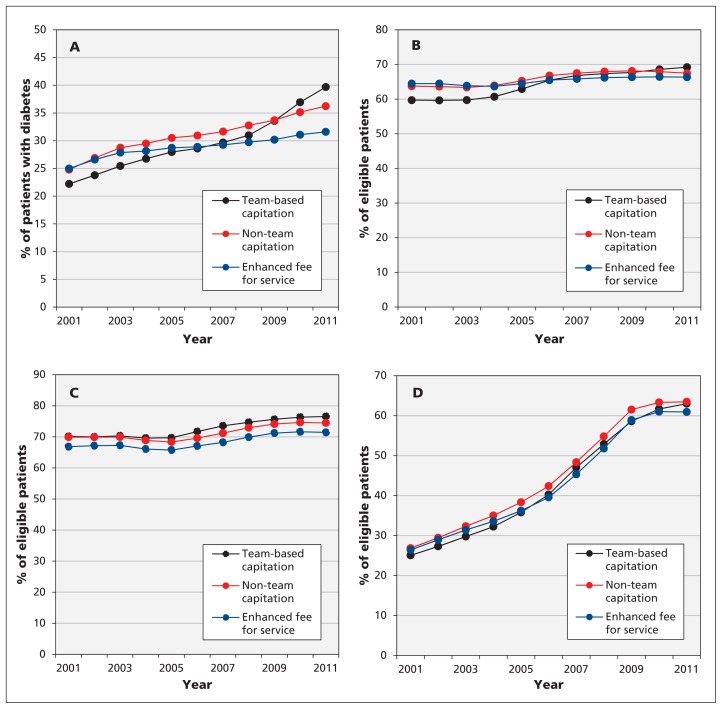
Patients with diabetes who were enrolled in team-based capitation practices in 2011 experienced the greatest improvements in recommended testing between 2001 and 2011 relative to other models (absolute difference in improvement 6.4% [95% CI 3.8% to 9.1%] compared with non–team-based capitation; 10.6% [95% CI 7.9% to 13.2%] compared with enhanced fee-for-service) (Figure 1A, Table 4). Patients enrolled in non–team-based capitation practices experienced greater improvement in recommended testing for diabetes over time than patients in enhanced fee-for-service practices (absolute difference in improvement 4.1%, 95% CI 1.5% to 6.8%). Patients enrolled in team-based capitation practices in 2011 also experienced the greatest improvements in cervical cancer screening between 2001 and 2011 (absolute difference in improvement 5.3% [95% CI 3.8% to 6.8%] compared with non–team-based capitation; 7.0% [95% CI 5.5% to 8.5%] compared with enhanced fee-for-service), with the largest improvements occurring between 2004 and 2006 (Figure 1B). There were no statistically significant differences in changes over time between patients in different payment models for breast and colorectal cancer screening (Figure 1C and 1D, Table 4).
Figure 1:
Provision of chronic disease management and prevention between 2001 and 2011, stratified by type of physician payment model in which patients were enrolled in 2011. (A) Recommended testing for diabetes mellitus, as percentage of patients with diabetes who received 1 retinal eye exam, 4 hemoglobin A1C tests and 1 cholesterol test in the 24 months before Mar. 31 of the fiscal year. (B) Cervical cancer screening, as percentage of women aged 35 to 69 years who received a Papanicolaou smear in the 30 months before Mar. 31 of the fiscal year, excluding those who underwent hysterectomy. (C) Breast cancer screening, as percentage of women aged 50 to 69 years who underwent mammography in the 30 months before Mar. 31 of the fiscal year, excluding those who underwent mastectomy or received treatment for breast cancer. (D) Colorectal cancer screening, as percentage of adults aged 50 to 74 years who either underwent fecal occult blood testing in the 24 months before Mar. 31 of the fiscal year or who had colonoscopy in the previous 10 years, excluding those who had colon cancer.
Table 4:
Comparison of changes in chronic disease management and prevention over the period 2001 to 2011, by type of payment model
| Comparison | Type of chronic disease management or prevention; absolute difference in improvement over time, % (95% CI)* | |||
|---|---|---|---|---|
| Recommended testing for diabetes | Cervical cancer screening | Breast cancer screening | Colorectal cancer screening | |
| Team-based capitation v. non–team-based capitation | 6.4 (3.8 to 9.1) | 5.3 (3.8 to 6.8) | 1.3 (−1.2 to 3.9) | 1.3 (−2.1 to 4.8) |
| Team-based capitation v. enhanced fee for service | 10.6 (7.9 to 13.2) | 7.0 (5.5 to 8.5) | 1.7 (−0.9 to 4.2) | 2.8 (−0.6 to 6.2) |
| Non–team-based capitation v. enhanced fee for service | 4.1 (1.5 to 6.8) | 1.7 (0.2 to 3.2) | 0.3 (−2.2 to 2.9) | 1.4 (−2.0 to 4.9) |
Note: CI = confidence interval.
The absolute difference in improvement over time is based on results from the fitted model and is expressed as a percentage.
Interpretation
We found that patients whose physicians were paid by capitation and worked in an interprofessional team setting experienced the greatest improvement in recommended testing for diabetes over time and were more likely to receive recommended testing for diabetes than patients whose physicians were paid by capitation but did not work in a team setting or whose physicians were paid by fee for service. Patients in a non–team-based capitation practice were more likely to experience improvements over time and to receive recommended testing for diabetes than those in a fee-for-service practice. There were no differences in cervical cancer screening between practice types, and most of the relative improvement occurred before transitions to new payment schemes. There were minimal differences in breast and colorectal cancer screening between practice types, and the relative differences predated changes in physician payment.
Our findings suggest that the shift to capitation payment and the addition of nonphysician health professionals to the care team have led to moderate improvements in processes of diabetes care, but the effects on cancer screening are less clear. Our findings are consistent with cross-sectional studies from Ontario showing that patients who saw fee-for-service physicians had the largest gaps in diabetes care16 but that little of the difference in preventive screening could be attributed to the type of physician payment.29 Our study builds on the longitudinal analysis by Kralj and Kantarevic,30 who found that physicians who transitioned from fee-for-service payment to capitation were 7% to 10% more likely to achieve cancer screening targets than those remaining in fee-for-service practice; however, in contrast to our study, theirs was a physician-level analysis and did not assess the relative influence of team-based care. Evidence from the US is mixed, whereby some studies have shown that practices that adopted components of patient-centred medical homes were more likely to provide better preventive services11,31–34 and chronic disease care,11,33,34 whereas other studies showed that the rate of improvement in quality of care was no greater in patient-centred medical homes than in comparison practices.13,35 These studies did not separate the effect of payment reform from other types of patient-centred medical home reform, such as the addition of nonphysician team members.
There is strong evidence that team-based care can improve both processes of care and outcomes for diabetes.10,36 Team-based practices in Ontario had the added advantage of participating in quality improvement learning collaboratives between 2008 and 2010 that were not available to other practices and that focused specifically on improving diabetes care and colorectal cancer screening.37 Adding primary care teams may be less important for cancer screening than other improvement strategies.38 Clinicians, policy-makers and the public will need to decide if the improvements in care noted in evaluations of Ontario’s reforms justify the related increase in primary care expenditure, from about $2.8 billion in 2006/07 to $3.7 billion in 2009/10.39 Weighing improvements against related costs is particularly difficult given that participation in reforms was voluntary and given that the physicians who transitioned to capitation and team-based models may have been those with a greater interest in chronic disease management and prevention.
Limitations
Our study had 3 major limitations. First, we were unable to assess important aspects of quality of care, such as timely access and patient satisfaction, nor did we have access to laboratory values or blood pressure data. Second, we were unable to infer causation. We evaluated a natural health policy experiment in which transition to a medical home was voluntary, with physicians and patients transitioning among several types of medical homes at different points in time. These complexities limited our choice of study design, necessitating a stratification and look-back method instead of a more traditional prospective cohort or interrupted time series design. Finally, there were important differences in patient and physician characteristics among types of medical home, and these may have influenced our findings, despite our attempts to control for differences with regression modelling. For example, patients whose physicians were paid primarily through fee for service were less likely to receive recommended testing for diabetes, but they were also more likely to be new immigrants and to have multiple comorbidities, factors that may pose challenges to care. These differences are partly explained by capitation payments that adjust for patient age and sex but not morbidity.14
Conclusion
Our findings suggest that the shift to capitation payment and the addition of team-based care in Ontario have led to moderate improvements in diabetes care, but the effects on cancer screening are less clear. Improvements in diabetes care were most marked when capitation payment to physicians was coupled with interprofessional care teams. We conclude that physician payment reform and team-based care have the potential to improve chronic disease management and prevention.
Acknowledgements:
The authors thank Don Redelmeier (Department of Medicine, Sunnybrook Health Sciences Centre, University of Toronto) and Irfan Dhalla (Department of Medicine, St. Michael’s Hospital, University of Toronto) for providing presubmission feedback on this manuscript.
Footnotes
Competing interests: Tara Kiran is the Board Chair for the St. Michael’s Hospital Academic Family Health Team. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Tara Kiran and Richard Glazier conceived of the study, and all authors contributed to designing the study. Alexander Kopp and Rahim Moineddin conducted the data analysis, and all authors interpreted the data. Tara Kiran drafted the manuscript, and all authors critically reviewed it. All authors read and approved the final manuscript, and all agree to act as guarantors of the work.
Funding: Funding was provided by Project 177411 from the Canadian Institutes of Health Research. The funding agency had no role in the design or conduct of the study; the collection, management, analysis or interpretation of the data; or the preparation, review or approval of the manuscript. This study was also supported by the Institute for Clinical Evaluative Sciences, which is funded by annual grants from the Ontario Ministry of Health and Long-Term Care. The opinions, results and conclusions reported in this article are those of the authors and are independent from these funding sources. No endorsement by the Institute for Clinical Evaluative Sciences or the Ontario Ministry of Health and Long-Term Care is intended or should be inferred. Parts of this material are based on data and information compiled and provided by Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed herein are those of the authors and not necessarily those of CIHI.
Tara Kiran is supported as a Clinician Investigator and Richard Glazier as a Clinician Scientist by the Department of Family and Community Medicine at the University of Toronto and at St. Michael’s Hospital.
References
- 1.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83:457–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips RL, Pugno PA, Saultz JW, et al. Health is primary: family medicine for America’s health. Ann Fam Med 2014;12(Suppl 1):S1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder SA, Frist W. Phasing out fee-for-service payment. N Engl J Med 2013;368:2029–32. [DOI] [PubMed] [Google Scholar]
- 4.Bodenheimer T, Ghorob A, Willard-Grace R, et al. The 10 building blocks of high-performing primary care. Ann Fam Med 2014;12:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landon BE. Structuring payments to patient-centered medical homes. JAMA 2014;312:1633–4. [DOI] [PubMed] [Google Scholar]
- 6.Edwards ST, Landon BE. Medicare’s chronic care management payment — payment reform for primary care. N Engl J Med 2014; 371:2049–51. [DOI] [PubMed] [Google Scholar]
- 7.Joint principles of the patient-centered medical home. Washington (DC): Patient Centered Primary Care Collaborative; 2007. Available: www.aafp.org/dam/AAFP/documents/practice_management/pcmh/initiatives/PCMHJoint.pdf (accessed 2015 Mar. 31). [Google Scholar]
- 8.Barr MS. The need to test the patient-centered medical home. JAMA 2008;300:834–5. [DOI] [PubMed] [Google Scholar]
- 9.Grumbach K, Bodenheimer T. A primary care home for Americans: putting the house in order. JAMA 2002;288:889–93. [DOI] [PubMed] [Google Scholar]
- 10.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA 2002;288:1909–14. [DOI] [PubMed] [Google Scholar]
- 11.Jaén CR, Ferrer RL, Miller WL, et al. Patient outcomes at 26 months in the patient-centered medical home National Demonstration Project. Ann Fam Med 2010;8 Suppl 1:S57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid RJ, Coleman K, Johnson EA, et al. The Group Health medical home at year two: cost savings, higher patient satisfaction, and less burnout for providers. Health Aff (Millwood) 2010; 29:835–43. [DOI] [PubMed] [Google Scholar]
- 13.Friedberg MW, Schneider EC, Rosenthal MB, et al. Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA 2014;311:815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glazier RH, Redelmeier DA. Building the patient-centered medical home in Ontario. JAMA 2010;303:2186–7. [DOI] [PubMed] [Google Scholar]
- 15.Hutchison B, Glazier R. Ontario’s primary care reforms have transformed the local care landscape, but a plan is needed for ongoing improvement. Health Aff (Millwood) 2013;32:695–703. [DOI] [PubMed] [Google Scholar]
- 16.Liddy C, Singh J, Hogg W, et al. Comparison of primary care models in the prevention of cardiovascular disease — a cross sectional study. BMC Fam Pract 2011;12:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiran T, Victor JC, Kopp A, et al. The relationship between primary care models and processes of diabetes care in Ontario. Can J Diabetes 2014;38:172–8. [DOI] [PubMed] [Google Scholar]
- 18.Population by year, by province and territory. Ottawa: Statistics Canada; 2014. Available: www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo02a-eng.htm (accessed 2015 Jan 20). [Google Scholar]
- 19.Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512–6. [DOI] [PubMed] [Google Scholar]
- 20.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2008;32:S1–201. [DOI] [PubMed] [Google Scholar]
- 21.Kiran T, Wilton AS, Moineddin R, et al. Effect of payment incentives on cancer screening in Ontario primary care. Ann Fam Med 2014;12:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kralj B. Measuring “rurality” for purposes of health-care planning: an empirical measure for Ontario. Toronto: Ontario Medical Association; 2005. [Google Scholar]
- 23.Steele LS, Glazier R, Lin E, et al. Using administrative data to measure ambulatory mental health service provision in primary care. Med Care 2004;42:960–5. [DOI] [PubMed] [Google Scholar]
- 24.The Johns Hopkins ACG System. Baltimore: The Johns Hopkins University; 2012. Available: www.acg.jhsph.org/ (accessed 2015 Jan 6). [Google Scholar]
- 25.McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003;157:940–3. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard AE, Ahern J, Fleischer NL, et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology 2010;21:467–74. [DOI] [PubMed] [Google Scholar]
- 27.Long J, Ryoo J. Using fractional polynomials to model non-linear trends in longitudinal data. Br J Math Stat Psychol 2010;63: 177–203. [DOI] [PubMed] [Google Scholar]
- 28.Tan Q, Thomassen M, Hjelmborg JvB, et al. A growth curve model with fractional polynomials for analysing incomplete time-course data in microarray gene expression studies. Adv Bioinformatics 2011;2011:261514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahrouge S, Hogg WE, Russell G, et al. Impact of remuneration and organizational factors on completing preventive manoeuvres in primary care practices. CMAJ 2012;184:E135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kralj B, Kantarevic J. Quality and quantity in primary care mixed-payment models: evidence from family health organizations in Ontario. Can J Econ 2013;46:208–38. [Google Scholar]
- 31.Ferrante JM, Balasubramanian BA, Hudson SV, et al. Principles of the patient-centered medical home and preventive services delivery. Ann Fam Med 2010;8:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson GL, Powers BJ, Chatterjee R, et al. The patient-centered medical home: a systematic review. Ann Intern Med 2013; 158: 169–78. [DOI] [PubMed] [Google Scholar]
- 33.Nelson KM, Helfrich C, Sun H, et al. Implementation of the patient-centered medical home in the Veterans Health Administration: associations with patient satisfaction, quality of care, staff burnout, and hospital and emergency department use. JAMA Intern Med 2014;174:1350–8. [DOI] [PubMed] [Google Scholar]
- 34.Phillips RL, Han M, Petterson SM, et al. Cost, utilization, and quality of care: an evaluation of Illinois’ Medicaid primary care case management program. Ann Fam Med 2014;12:408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solberg LI, Asche SE, Fontaine P, et al. Trends in quality during medical home transformation. Ann Fam Med 2011;9:515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet 2012;379:2252–61. [DOI] [PubMed] [Google Scholar]
- 37.Harris SB, Green ME, Brown JB, et al. Impact of a quality improvement program on primary healthcare in Canada: a mixed-method evaluation. Health Policy 2015;119:405–16. [DOI] [PubMed] [Google Scholar]
- 38.Weller DP, Patnick J, McIntosh HM, et al. Uptake in cancer screening programmes. Lancet Oncol 2009;10:693–9. [DOI] [PubMed] [Google Scholar]
- 39.Funding alternatives for family physicians [Chapter 3, Section 3.06]. In: Annual report of the Office of the Auditor General of Ontario. Toronto: Ministry of Health and Long-Term Care; 2011. p. 150–70. [Google Scholar]