Fig. 3. Rigor binding actin affinity of wild type and R403Q human β-cardiac sS1.
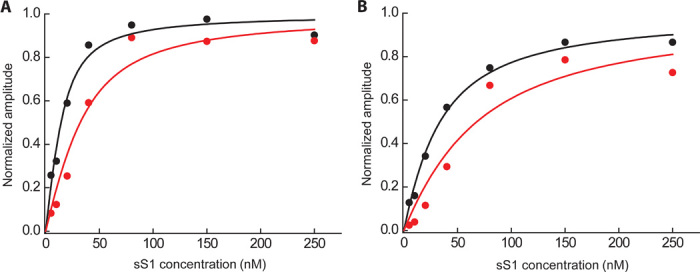
(A and B) Phalloidin-stabilized actin was incubated with various amounts of wild-type (black) and R403Q (red) human β-cardiac sS1 before mixing with 20 μM ATP at either (A) 25 mM KCl or (B) 100 mM KCl. The binding experiment at 25 mM ionic strength (A) was repeated at 100 mM ionic strength (B) to ensure that the changes observed were not due to nonspecific interactions. The pyrene fluorescence of the free actin is quenched upon binding of the myosin. Hence, the amount of actin that is tightly bound to myosin at equilibrium can be determined by observing the amplitude change of increase in fluorescence after rapidly mixing the equilibrium actin-sS1 mixture with ATP, which dissociates the actin·sS1 complex. This amplitude change as a function of myosin concentration, starting with a fixed concentration of actin, generates a curve that reflects the equilibrium dissociation constant of the binding (KA; see table S1). A single exponential function was fit to the raw fluorescence trace, and the amplitude from the fit was plotted as a function of different sS1 concentrations. One representative experimental curve is shown in (A) and (B). The best fit of the amplitude dependence on sS1 concentration was determined using the quadratic equation describing the binding isotherm as before (34, 36).
