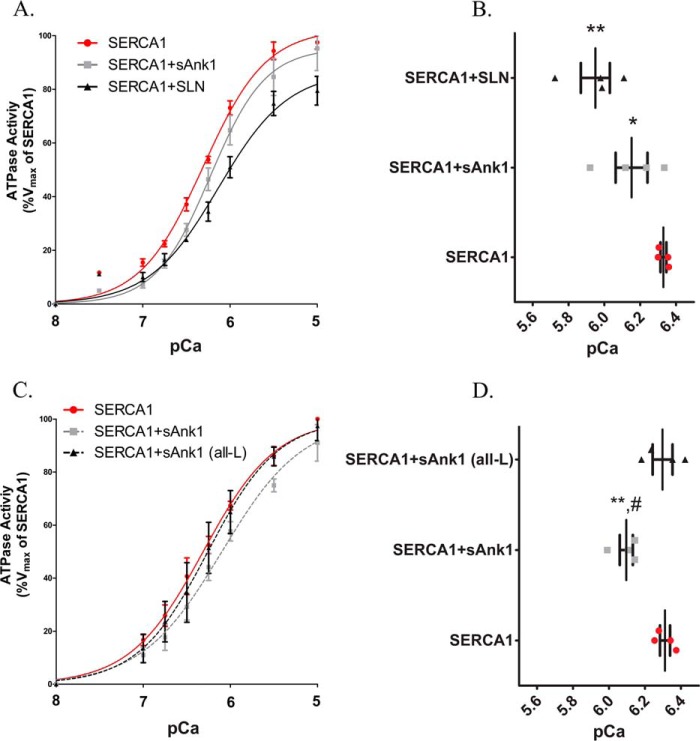
FIGURE 6.
Ca2+-ATPase assay in COS7 microsomes. A, COS7 cells were transfected with the indicated cDNA construct(s). ATPase activity was determined at each [Ca2+]free compared with the Vmax measured for SERCA1 alone, following normalization of the levels of SERCA1 expression as determined by immunoblotting (see “Experimental Procedures”). Data were fitted to the equation for a general cooperative model for substrate binding. Results show that co-expression of sAnk1 with SERCA leads to a reduction of the apparent affinity of SERCA for Ca2+ but that the effect of sAnk1 is less than that of SLN. B, the KCa2+ ([Ca2+]free required for half-maximal activation) values were determined from each curve. Mean KCa2+ values were as follows: SERCA1, pCa = 6.33 (468 nm); SERCA1 + sAnk1, pCa = 6.15 (708 nm); SERCA1 + SLN, pCa = 5.95 (1122 nm). C, unlike sAnk1 (WT), sAnk1 (all-L) did not significantly alter the affinity of SERCA1 for Ca2+. D, mean KCa2+ values were as follows: SERCA1, pCa = 6.31 (488 nm); SERCA1 + sAnk1, pCa = 6.10 (794 nm); SERCA1 + sAnk1 (all-L), pCa = 6.30 (502 nm). Statistics used one-way analysis of variance. *, p < 0.05 versus SERCA1; **, p < 0.01 versus SERCA1; #, p < 0.05 versus SERCA1 + sAnk1 (all-L). Error bars, S.E.