Figure 4.
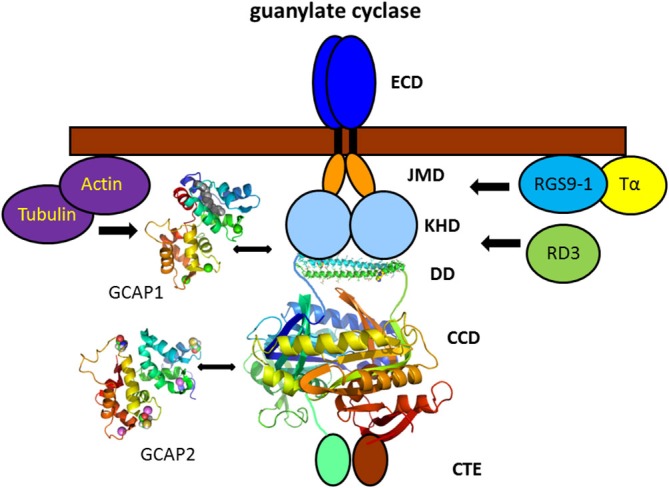
Vertebrate photoreceptor GC and interacting proteins. Photoreceptor GCs contain several domains denoted as extracellular domain (ECD, which in rod outer segment is present in the intradiscal lumen; TM, transmembrane domain; JMD, juxtamembrane domain; KHD, kinase homology domain; DD, dimerization domain; CCD, cyclase catalytic domain; and CTE, a C-terminal extension). The tertiary structure of the DD and CCD is adapted from the solved three-dimensional structure of soluble GCs (Ma et al., 2010; Allerston et al., 2013) that display a high sequence homology with membrane bound GCs in these domains (PDB codes: 3HLS and 3UVJ). Assembly and topography of GC domains, in particular of the DD and CCD is arbitrarily chosen. GCAP1 and GCAP2 activate the target GC at low cytoplasmic Ca2+-concentration bringing the cell back to the dark state. The exact regions of interaction and/or regulation by GCAPs are a matter of debate (see main text). Interaction with other proteins was shown by biochemical procedures, but physiological meaning is lacking so far. Structures of GCAP1 and GCAP2 are based on the published x-ray and NMR-structure, respectively (GCAP1: 2R2I, Stephen et al., 2007; GCAP2: 1JBA, Ames et al., 1999).
