FIGURE 3.
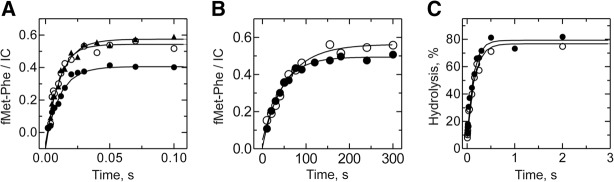
Reactions with natural substrates. (A) PT with cognate aa-tRNA was measured upon mixing wt (open circles), L27Δ1-6 (closed triangles), or ΔL27 (closed circles) ICs (50 nM) containing fMet-tRNAfMet in the P site and a UUC codon in the A site with TC-Phe (10 µM). Solid lines represent the results of exponential fitting of the time points, which yielded the rates of 110 ± 13, 90 ± 9, and 90 ± 8 sec−1 for wt, L27Δ1-6, and ΔL27 ribosomes, respectively. (B) PT with near cognate aa-tRNA was measured as in A, using ICs with a CUC codon in the A site. Exponential fitting (solid lines) yielded the rates of 0.017 ± 0.002 and 0.024 ± 0.002 sec−1 for wt and ΔL27 ribosomes, respectively. (C) Peptide release was measured upon mixing wt (open circles) or ΔL27 (closed circles) ICs (75 nM) programmed on a UAA codon with RF2 (1.5 µM). The rates, determined by exponential fitting (solid lines), were 6.6 ± 0.6 and 7.6 ± 0.8 sec−1 for wt and ΔL27 ribosomes, respectively.