Fig. 2.
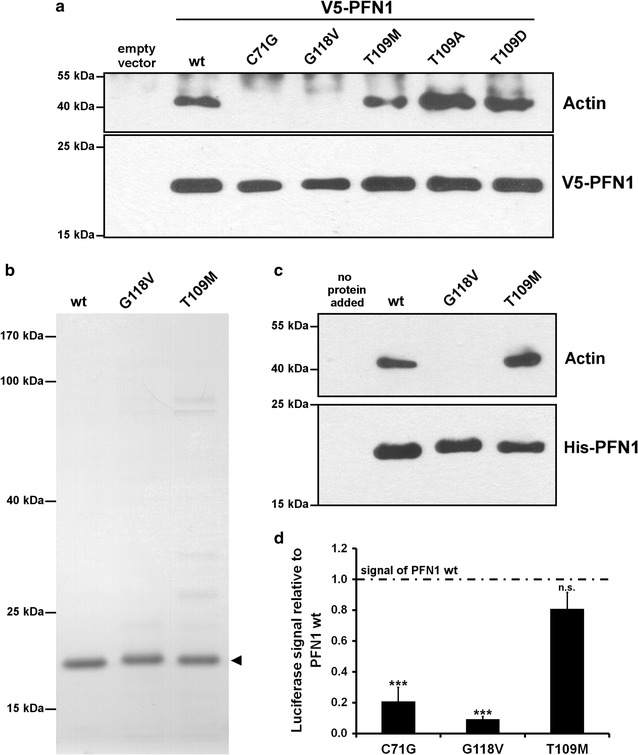
PFN1 T109M mutant protein is not impaired in actin binding. a Co-immunoprecipitation of lysates of HEK293 cells overexpressing wildtype and mutant V5-PFN1 using a V5-antibody followed by Western blot detection of V5-PFN1 and actin. b Purified recombinant His6-tagged wildtype and mutant PFN1 (arrowhead) used for the pulldown assay was subjected to SDS-PAGE followed by staining with coomassie brilliant blue. c His6-tag pulldown using recombinant His6-PFN1 and lysates of HEK293 cells. His6-tagged PFN1 and binding proteins were precipitated with Ni–NTA agarose (Qiagen) and subjected to Western blotting. d Quantification of the interaction of actin with wildtype and mutant PFN1 using a split luciferase assay. Luciferase activity from protein complementation was measured in HEK293 cells 24 h after transfection with plasmids coding for PFN1-hGluc(1) and actin-hGluc(2). Signals were normalized to the expression of both fusion proteins determined by Western blotting and are shown relative to the signal of wildtype PFN1-hGluc(1) and actin-hGluc(2) (n = 4; 2–7 measurements each; bars indicate mean ± SEM; *** p ≤ 0.001 in a two tailed student’s t test; n.s. = not significant)
