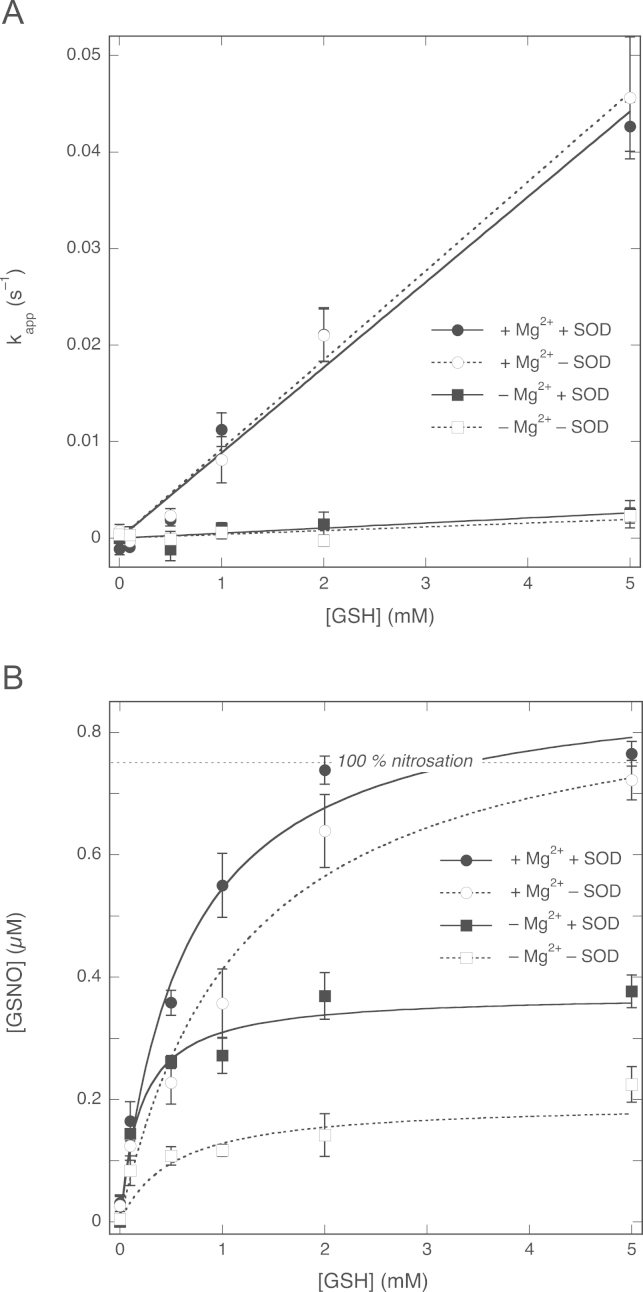
Fig. 6.
Effect of SOD on the [GSH] dependence of NO decay rate and GSNO formation. (A) Apparent pseudo-first-order rate constants as a function of the GSH concentration. The lines through the data points are best linear fits (y=ax) with a representing the apparent second-order rate constant. Fitting parameters: +Mg2+, +SOD, 8.8±0.4 M−1 s−1 (R=0.989); +Mg2+, −SOD, 9.2±0.3 M−1 s−1 (R=0.995); −Mg2+, +SOD, 0.52±0.13 M−1 s−1 (R=0.82); −Mg2+, −SOD, 0.39±0.10 M−1 s−1 (R=0.80). (B) GSNO yields for varying concentrations of GSH. The lines through the data points are best fits to the hyperbola y=bx/(a+x), where a represents the EC50 for GSH and b the maximal GSNO yield. Fitting parameters: +Mg2+, +SOD, EC50 0.64±0.08 mM, [GSNO]max 0.89±0.03 µM (R=0.993); +Mg2+, −SOD, EC50 1.2±0.2 mM, [GSNO]max 0.90±0.06 µM (R=0.987); −Mg2+, +SOD, EC50 0.20±0.05 mM, [GSNO]max 0.37±0.02 µM (R=0.998); −Mg2+, −SOD, EC50 0.5±0.3 mM, [GSNO]max 0.19±0.03 µM (R=0.973). Experimental conditions: 1 µM PROLI/NO, GSH as indicated, 4 mM CuSO4, 0 (white symbols) or 1000 (black symbols) U/ml SOD, 0.1 mM DTPA, 0 (squares) or 5 (circles) mM MgCl2, and 50 mM TEA (pH 7.4) at 37 °C. Data in the presence of SOD are from Fig. 3 and included for easier comparison. Data are shown ±SEM (n=5).