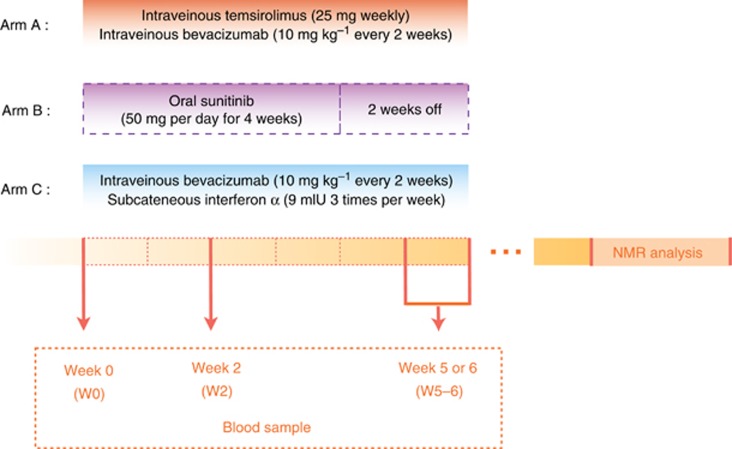
Figure 1.
Study Design of the TORAVA trial. Patients with untreated mRCC were randomised using a 2:1:1 ratio: arm A was administered a combination of bevacizumab and temsirolimus; arm B was treated with sunitinib; arm C received a combination of interferon-α and bevacizumab. Arm A is the experimental arm and the two others arms (B and C) are standard first-line treatments of mRCC. Blood samples were collected at three different times: at baseline (W0), that is, before the first therapy cure; 2 weeks after the start of treatment (W2); and 5–6 weeks after beginning of treatment (W5–6). NMR analyses were performed after completion of the clinical trial.