Abstract
Structure-activity studies demonstrate that galanin fragments 1-15 and 2-29 are fully active, whereas fragment 3-29 has been reported to be inactive, in a number of different in vivo models. M15, a chimeric peptide comprising galanin 1-13 and substance P5-11, has recently been found to be a potent galanin antagonist. Direct effects of galanin at the level of the pituitary have been defined, yet, paradoxically, a number of studies have been unable to demonstrate galanin binding to an anterior pituitary receptor. Porcine galanin stimulated prolactin release from dispersed rat anterior pituitary cells up to 180% +/- 12% (mean +/- SEM) of control secretion. The addition of a specific galanin antiserum caused a profound inhibition of basal prolactin release, maximal inhibition being 12% +/- 0.5% of control secretion. Addition of M15 produced no effect on basal or galanin-stimulated prolactin release. Galanin fragment 3-29 was fully active when compared to galanin 1-29. Fragments 5-29 and 8-29 stimulated prolactin release to a lesser extent and galanin 1-15, 10-29, and 20-29 had no significant prolactin-releasing activity. Using [mono(125I)iodo-Tyr26]galanin or porcine 125I-labeled Bolton-Hunter [mono(125I)iodo-Lys25]galanin, no anterior pituitary membrane binding was observed. In contrast, 125I-labeled Bolton-Hunter N-terminally labeled galanin allowed characterization of a single high-affinity anterior pituitary galanin receptor with a Kd of 4.4 +/- 0.34 nM and a Bmax of 79 +/- 8.3 fmol/mg of protein. The IC50 for porcine galanin was 0.51 +/- 0.04 nM but for M15 was in excess of 10 microM. Galanin 3-29 fully displaced the label with an IC50 of 0.96 +/- 0.7 nM. The IC50 for galanin 5-29 was 200 nM, whereas 8-29 and 1-15 were > 1 microM. Galanin 10-29 and 20-29 failed to displace the label. These data suggest the presence of a high-affinity pituitary galanin receptor, designated GAL-R2, in which region 3-10 and amino acid 25 are crucial for membrane binding and biological activity, in contrast to the known gut/brain galanin receptor (designated GAL-R1). A number of tissues known to bind or respond to galanin were screened. GAL-R2 would appear to be expressed only in the anterior pituitary and hypothalamus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartfai T., Bedecs K., Land T., Langel U., Bertorelli R., Girotti P., Consolo S., Xu X. J., Wiesenfeld-Hallin Z., Nilsson S. M-15: high-affinity chimeric peptide that blocks the neuronal actions of galanin in the hippocampus, locus coeruleus, and spinal cord. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10961–10965. doi: 10.1073/pnas.88.23.10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet W. M., Hill S. F., Ghatei M. A., Bloom S. R. Galanin in the normal human pituitary and brain and in pituitary adenomas. J Endocrinol. 1991 Sep;130(3):463–467. doi: 10.1677/joe.0.1300463. [DOI] [PubMed] [Google Scholar]
- Bhogal R., Smith D. M., Bloom S. R. Investigation and characterization of binding sites for islet amyloid polypeptide in rat membranes. Endocrinology. 1992 Feb;130(2):906–913. doi: 10.1210/endo.130.2.1310282. [DOI] [PubMed] [Google Scholar]
- Ch'ng J. L., Christofides N. D., Anand P., Gibson S. J., Allen Y. S., Su H. C., Tatemoto K., Morrison J. F., Polak J. M., Bloom S. R. Distribution of galanin immunoreactivity in the central nervous system and the responses of galanin-containing neuronal pathways to injury. Neuroscience. 1985 Oct;16(2):343–354. doi: 10.1016/0306-4522(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Chen Y., Couvineau A., Laburthe M., Amiranoff B. Solubilization and molecular characterization of active galanin receptors from rat brain. Biochemistry. 1992 Mar 3;31(8):2415–2422. doi: 10.1021/bi00123a029. [DOI] [PubMed] [Google Scholar]
- Davis T. M., Burrin J. M., Bloom S. R. Growth hormone (GH) release in response to GH-releasing hormone in man is 3-fold enhanced by galanin. J Clin Endocrinol Metab. 1987 Dec;65(6):1248–1252. doi: 10.1210/jcem-65-6-1248. [DOI] [PubMed] [Google Scholar]
- Delvaux M., Botella A., Fioramonti J., Frexinos J., Bueno L. Galanin induces contraction of isolated cells from circular muscle layer of pig ileum. Regul Pept. 1991 Feb 26;32(3):369–374. doi: 10.1016/0167-0115(91)90030-k. [DOI] [PubMed] [Google Scholar]
- Fisone G., Berthold M., Bedecs K., Undén A., Bartfai T., Bertorelli R., Consolo S., Crawley J., Martin B., Nilsson S. N-terminal galanin-(1-16) fragment is an agonist at the hippocampal galanin receptor. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9588–9591. doi: 10.1073/pnas.86.23.9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisone G., Langel U., Carlquist M., Bergman T., Consolo S., Hökfelt T., Undén A., Andell S., Bartfai T. Galanin receptor and its ligands in the rat hippocampus. Eur J Biochem. 1989 Apr 15;181(1):269–276. doi: 10.1111/j.1432-1033.1989.tb14721.x. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Brooks B., McDonald T. J., Barnett W., Kostolanska F., Yanaihara C., Yanaihara N., Rökaeus A. Actions of galanin fragments on rat, guinea-pig, and canine intestinal motility. Peptides. 1988 Sep-Oct;9(5):1183–1189. doi: 10.1016/0196-9781(88)90105-2. [DOI] [PubMed] [Google Scholar]
- Gabriel S. M., Milbury C. M., Nathanson J. A., Martin J. B. Galanin stimulates rat pituitary growth hormone secretion in vitro. Life Sci. 1988;42(20):1981–1986. doi: 10.1016/0024-3205(88)90497-3. [DOI] [PubMed] [Google Scholar]
- Gaymann W., Falke N. Galanin lacks binding sites in the porcine pituitary and has no detectable effect on oxytocin and vasopressin release from rat neurosecretory endings. Neurosci Lett. 1990 Apr 20;112(1):114–119. doi: 10.1016/0304-3940(90)90332-4. [DOI] [PubMed] [Google Scholar]
- Ghigo E., Maccario M., Arvat E., Valetto M. R., Valente F., Nicolosi M., Mazza E., Martina V., Cocchi D., Camanni F. Interactions of galanin and arginine on growth hormone, prolactin, and insulin secretion in man. Metabolism. 1992 Jan;41(1):85–89. doi: 10.1016/0026-0495(92)90195-g. [DOI] [PubMed] [Google Scholar]
- Gregersen S., Hermansen K., Langel U., Fisone G., Bartfai T., Ahrén B. Galanin-induced inhibition of insulin secretion from rat islets: effects of rat and pig galanin and galanin fragments and analogues. Eur J Pharmacol. 1991 Oct 2;203(1):111–114. doi: 10.1016/0014-2999(91)90797-t. [DOI] [PubMed] [Google Scholar]
- Gregersen S., Hermansen K., Yanaihara N., Ahrén B. Galanin fragments and analogues: effects on glucose-stimulated insulin secretion from isolated rat islets. Pancreas. 1991 Mar;6(2):216–220. [PubMed] [Google Scholar]
- Hedlund P., Fuxe K. Chronic imipramine treatment increases the affinity of [125I]galanin binding sites in the tel- and diencephalon of the rat and alters the 5-HT1A/galanin receptor interaction. Acta Physiol Scand. 1991 Jan;141(1):137–138. doi: 10.1111/j.1748-1716.1991.tb09058.x. [DOI] [PubMed] [Google Scholar]
- Hulting A. L., Meister B., Carlsson L., Hilding A., Isaksson O. On the role of the peptide galanin in regulation of growth hormone secretion. Acta Endocrinol (Copenh) 1991 Nov;125(5):518–525. doi: 10.1530/acta.0.1250518. [DOI] [PubMed] [Google Scholar]
- Inoue T., Kato Y., Koshiyama H., Yanaihara N., Imura H. Galanin stimulates the release of vasoactive intestinal polypeptide from perifused hypothalamic fragments in vitro and from periventricular structures into the cerebrospinal fluid in vivo in the rat. Neurosci Lett. 1988 Feb 15;85(1):95–100. doi: 10.1016/0304-3940(88)90435-1. [DOI] [PubMed] [Google Scholar]
- Kaplan L. M., Gabriel S. M., Koenig J. I., Sunday M. E., Spindel E. R., Martin J. B., Chin W. W. Galanin is an estrogen-inducible, secretory product of the rat anterior pituitary. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7408–7412. doi: 10.1073/pnas.85.19.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama H., Kato Y., Inoue T., Murakami Y., Ishikawa Y., Yanaihara N., Imura H. Central galanin stimulates pituitary prolactin secretion in rats: possible involvement of hypothalamic vasoactive intestinal polypeptide. Neurosci Lett. 1987 Mar 20;75(1):49–54. doi: 10.1016/0304-3940(87)90073-5. [DOI] [PubMed] [Google Scholar]
- Kuwahara A., Ozaki T., Yanaihara N. Structural requirements for galanin action in the guinea-pig ileum. Regul Pept. 1990 Jun;29(1):23–29. doi: 10.1016/0167-0115(90)90106-7. [DOI] [PubMed] [Google Scholar]
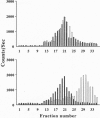
- Land T., Langel U., Bartfai T. Hypothalamic degradation of galanin(1-29) and galanin(1-16): identification and characterization of the peptidolytic products. Brain Res. 1991 Sep 6;558(2):245–250. doi: 10.1016/0006-8993(91)90775-q. [DOI] [PubMed] [Google Scholar]
- Land T., Langel U., Löw M., Berthold M., Undén A., Bartfai T. Linear and cyclic N-terminal galanin fragments and analogs as ligands at the hypothalamic galanin receptor. Int J Pept Protein Res. 1991 Sep;38(3):267–272. doi: 10.1111/j.1399-3011.1991.tb01438.x. [DOI] [PubMed] [Google Scholar]
- Loutradis D., Kallianidis K., Sakellaropoulos G., Dokos J., Siskos K., Creatsas G., Deligeoroglou E., Michalas S., Aravantinos D. Outcome of ovarian response after suppression with a gonadotropin releasing hormone agonist in different chronological periods prior to gonadotropin stimulation for in vitro fertilization. Gynecol Obstet Invest. 1991;32(2):68–71. doi: 10.1159/000292997. [DOI] [PubMed] [Google Scholar]
- López F. J., Merchenthaler I., Ching M., Wisniewski M. G., Negro-Vilar A. Galanin: a hypothalamic-hypophysiotropic hormone modulating reproductive functions. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4508–4512. doi: 10.1073/pnas.88.10.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
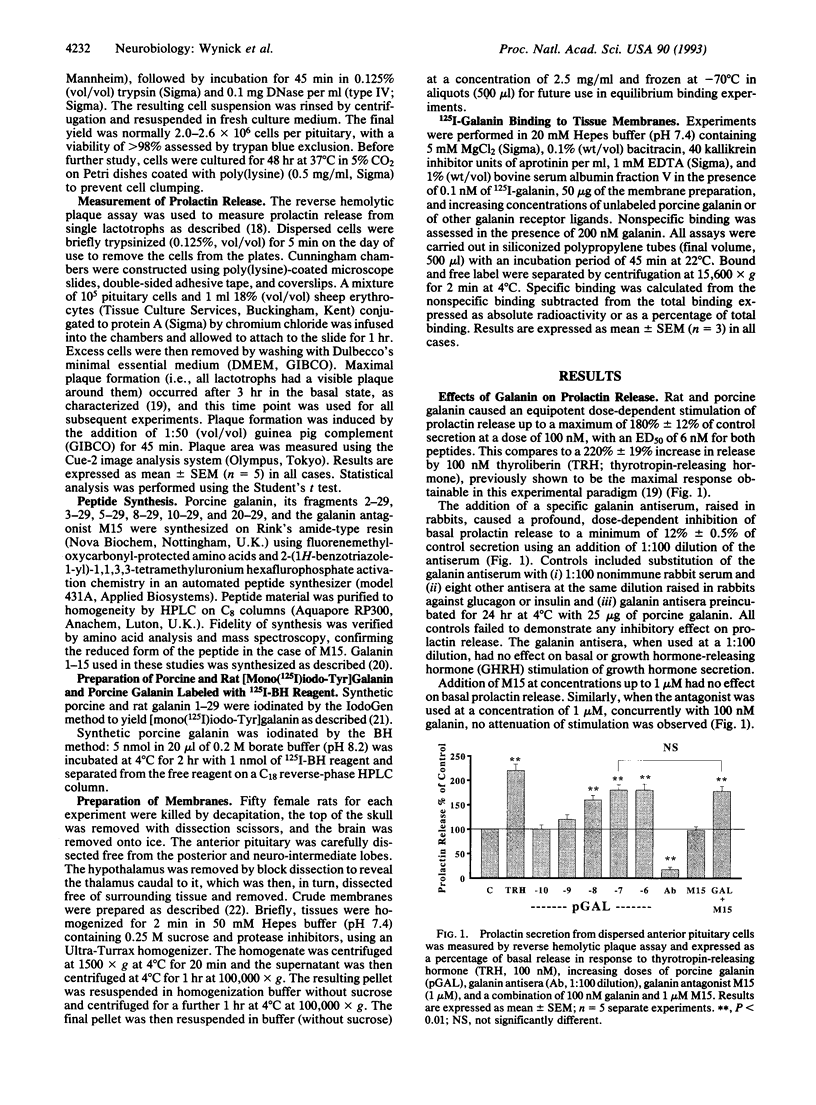
- Ottlecz A., Samson W. K., McCann S. M. Galanin: evidence for a hypothalamic site of action to release growth hormone. Peptides. 1986 Jan-Feb;7(1):51–53. doi: 10.1016/0196-9781(86)90060-4. [DOI] [PubMed] [Google Scholar]
- Rossowski W. J., Rossowski T. M., Zacharia S., Ertan A., Coy D. H. Galanin binding sites in rat gastric and jejunal smooth muscle membrane preparations. Peptides. 1990 Mar-Apr;11(2):333–338. doi: 10.1016/0196-9781(90)90089-n. [DOI] [PubMed] [Google Scholar]
- Rökaeus A., Melander T., Hökfelt T., Lundberg J. M., Tatemoto K., Carlquist M., Mutt V. A galanin-like peptide in the central nervous system and intestine of the rat. Neurosci Lett. 1984 Jun 15;47(2):161–166. doi: 10.1016/0304-3940(84)90423-3. [DOI] [PubMed] [Google Scholar]
- Sahu A., Crowley W. R., Tatemoto K., Balasubramaniam A., Kalra S. P. Effects of neuropeptide Y, NPY analog (norleucine4-NPY), galanin and neuropeptide K on LH release in ovariectomized (ovx) and ovx estrogen, progesterone-treated rats. Peptides. 1987 Sep-Oct;8(5):921–926. doi: 10.1016/0196-9781(87)90081-7. [DOI] [PubMed] [Google Scholar]
- Servin A. L., Amiranoff B., Rouyer-Fessard C., Tatemoto K., Laburthe M. Identification and molecular characterization of galanin receptor sites in rat brain. Biochem Biophys Res Commun. 1987 Apr 14;144(1):298–306. doi: 10.1016/s0006-291x(87)80510-7. [DOI] [PubMed] [Google Scholar]
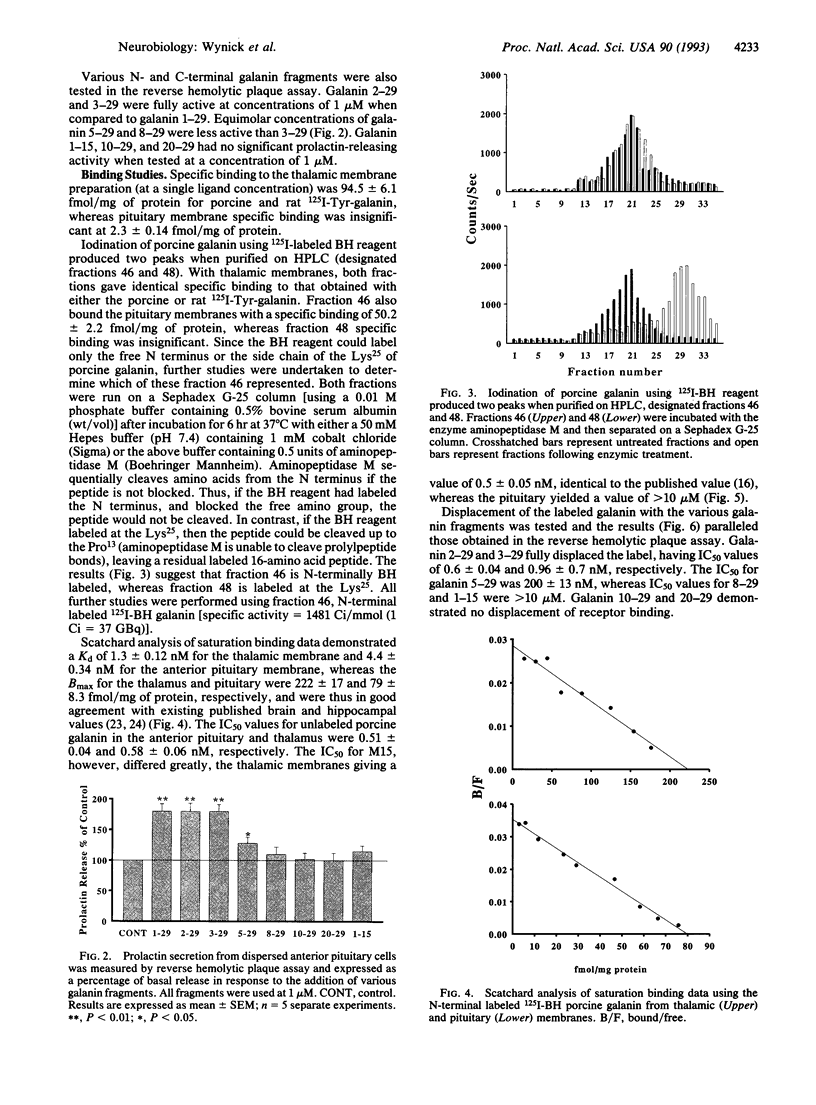
- Skofitsch G., Jacobowitz D. M. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985 May-Jun;6(3):509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Rökaeus A., Jörnvall H., McDonald T. J., Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983 Nov 28;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- Wynick D., Bloom S. R. Magnetic bead separation of anterior pituitary cells. Neuroendocrinology. 1990 Dec;52(6):560–565. doi: 10.1159/000125644. [DOI] [PubMed] [Google Scholar]
- Wynick D., Critchley R., Venetikou M. S., Burrin J. M., Bloom S. R. Purification of functional lactotrophs and somatotrophs from female rats using fluorescence-activated cell sorting. J Endocrinol. 1990 Aug;126(2):269–274. doi: 10.1677/joe.0.1260269. [DOI] [PubMed] [Google Scholar]
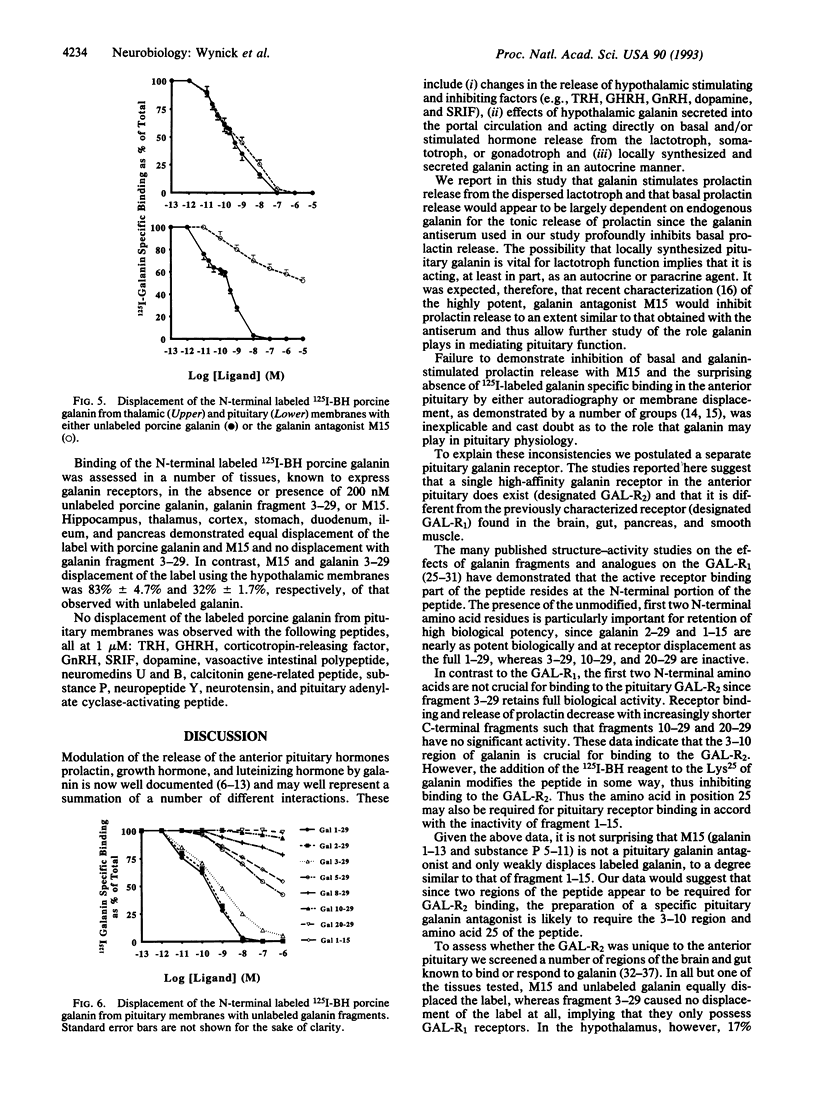
- Wynick D., Venetikou M. S., Critchley R., Burrin J. M., Bloom S. R. Flow cytometric analysis of functional anterior pituitary cells from female rats. J Endocrinol. 1990 Aug;126(2):261–268. doi: 10.1677/joe.0.1260261. [DOI] [PubMed] [Google Scholar]
- Xu X. J., Wiesenfeld-Hallin Z., Fisone G., Bartfai T., Hökfelt T. The N-terminal 1-16, but not C-terminal 17-29, galanin fragment affects the flexor reflex in rats. Eur J Pharmacol. 1990 Jun 21;182(1):137–141. doi: 10.1016/0014-2999(90)90502-w. [DOI] [PubMed] [Google Scholar]
- Yanaihara N., Yanaihara C., Zhang T., Hoshino M., Iguchi K., Mochizuki T. Recent advances in brain-gut hormones. Arch Histol Cytol. 1989;52 (Suppl):49–54. doi: 10.1679/aohc.52.suppl_49. [DOI] [PubMed] [Google Scholar]