Table 2.
Initial SAR data from benzomorpholinone hit expansion
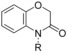 | ||||
|---|---|---|---|---|
| Compd | R | IC50 [μm][a] | LE[b] | |
| TbNMT | hNMT | TbNMT | ||
| 14 | CH2COOEt | 12 | >100 | 0.42 |
| 15 | CH(CH3)COOEt | >100 | >100 | – |
| 16 | CH2CH2COOEt | 13 | >100 | 0.38 |
| 17 | 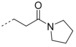 |
11 | >100 | 0.34 |
| 18 | CH2CH2CON(CH3)2 | 50 | >100 | – |
| 19 | CH2CON(CH3)2 | >100 | >100 | – |
| 20 | CH2CONHCH3 | >100 | >100 | – |
| 21 | 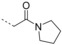 |
>100 | ND | – |
| 22 | 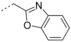 |
24 | >100 | 0.31 |
| 23 | 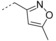 |
2.9 | >100 | 0.43 |
| 24 | 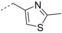 |
44 | >100 | 0.33 |
| 25 | 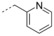 |
22 | >100 | 0.38 |
| 26 | 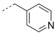 |
>100 | >100 | – |
| 27 | 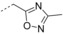 |
>100 | >100 | – |
| 28 | 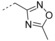 |
7.0 | >100 | 0.40 |
| 29 | 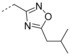 |
18 | >100 | 0.32 |
| 30 | 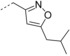 |
9 | 30 | 0.34 |
| 31 | 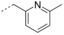 |
41 | 69 | 0.31 |
| 32 | 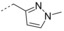 |
80 | >100 | – |
[a] Values shown are the mean of two or more determinations; ND=not determined. [b] Ligand efficiency (LE), determined for compounds with T. brucei NMT potency <50 μm, was calculated as 0.6 ln(IC50)/(heavy atom count).[11]
