Abstract
ACTH-secreting pituitary adenomas (Cushing’s disease, CD) are the most frequent cause of Cushing’s syndrome. To test whether the size of ACTH-secreting adenomas correlates with the degree of biochemical and clinical features of hypercortisolism, we retrospectively reviewed all newly diagnosed CD patients seen at our institution by two neuro-endocrinologists over a 10-year time period. We documented the number of clinical manifestations and baseline hormonal measurements. There were 37 microadenomas (μAs) and 16 macroadenomas (MAs). We sought to characterize the relationship between tumor size (μA vs. MA) and number of signs and symptoms of hypercortisolism and biochemical assessment of hypercortisolemia. There were no significant differences in mean age, BMI, or prevalence of hypertension and type 2 diabetes between the μA and MA groups. However, the MAs had fewer clinical manifestations of hypercortisolism (29.4% vs. 36.1%, P = 0.02) compared to μAs. There was a higher prevalence of easy bruisability and proximal muscle weakness in the μAs, but otherwise the prevalence of signs and symptoms were similar between groups. The MAs had a lower random serum cortisol (18.2 ± 2.4 vs. 25.9 ± 1.8 mcg/dl, P = 0.018), lower cortisol:ACTH ratio (0.25 ± 0.03 vs. 0.42 ± 0.05, P < 0.048), and lower cortisol:tumor diameter ratio (14.1 ± 2.2 vs. 56.8 ± 7.2, P < 0.0001) than the μAs. We conclude that tumor size does not directly correlate with the extent of hormonal activity in ACTH-secreting adenomas. Biochemical activity and clinical manifestations may be mild even in larger tumors, and therefore a high index of suspicion may be necessary to recognize hypercortisolism in pituitary MAs.
Keywords: Pituitary adenoma, Cushing, ACTH, Symptoms
Introduction
ACTH-secreting pituitary adenomas are considered the most common cause of endogenous hypercortisolemia, accounting for about two-thirds of cases of endogenous Cushing’s syndrome [1, 2]. The majority of ACTH-secreting pituitary adenomas are microadenomas (μAs), and do not cause mass effect symptoms [3]. They are usually identified on the basis of clinical features of hypercortisolism. Conversely, patients with macroadenomas (MA) of any type (hormone-secreting or non-functioning) may present with symptoms of mass effect (i.e. headache, visual field deficits, or ophthalmoplegia), and further characterization of the tumor type requires a careful clinical and biochemical evaluation. While Cushing’s disease (CD) itself is relatively rare, the occurrence of this condition due to a MA is thought to be even less common, accounting for 4–23% of cases [4–7].
The relationship between the size of a pituitary tumor and the severity of hormonal hypersecretion is well-established for growth-hormone- and prolactin-secreting tumors; in general, larger tumors have higher levels of hormone secretion [8–10]. Although ACTH-secreting MAs have in some series been reported to have higher baseline levels of ACTH and cortisol compared to μAs, the prevalence of clinical signs and symptoms of hypercortisolemia may not correlate with the size of the tumor [4, 11, 12]. Nonetheless, given the perceived relationship between tumor size and severity of clinical manifestations, if a patient with a MA has only mild features of cortisol excess, a clinician may elect not to pursue testing for hypercortisolemia. The goal of our study was to characterize the clinical and biochemical features of newly diagnosed CD patients in order to determine whether the extent of hypercortisolism is related to tumor size.
Patients and methods
With approval from our Institution’s IRB, we performed a retrospective electronic chart review of 53 newly diagnosed CD patients (16 MAs, 37 μAs) seen by two of the authors (R.S. and G.S.W.) in their Endocrinology clinical practices over a 10-year period (2000–2010). All of the patients were evaluated by one of the two practitioners prior to transsphenoidal surgery, and the majority of the cases were evaluated by one endocrinologist (R.S., who saw 76 and 88% of the μAs and MAs, respectively). Among 53 patients, 51 underwent pituitary surgery, while 2 μA patients are currently awaiting surgery. Among the 51 patients who underwent pituitary surgery, all but two of the surgeries were performed at our institution, and therefore follow-up data were available for 49 cases. We quantified the number of signs and symptoms of hyper-cortisolism, and mass effect as documented at the time of the initial endocrine consultation visit. If a sign or reported symptom was not mentioned in the clinic note, it was assumed to be absent. Depending on the gender of the patient, we assessed 14–15 signs, 19–22 symptoms or associated conditions, and overall 33–37 clinical manifestations (combined signs and symptoms). With the exception of the patients’ self-reported heights, all of the data was collected from the electronic medical records and patient charts. We sought to identify whether there were differences in biochemical or clinical parameters after sorting the tumors according to size (μA vs. MA).
The signs of hypercortisolism were quantified from the physical examination section of the clinic note. For each sign, any of the following descriptors were considered synonymous and indicative of a positive finding: (1) Obesity/cushingoid appearance (generalized/centripetal obesity, Cushingoid body habitus), (2) Facial fullness (moon/round facies, facial roundness), (3) Facial plethora (plethoric, facial redness, telangectasias), (4) Supraclavicular fat accumulation/fat pad, (5) Dorsocervical fat (retrocervical/posterior cervical fat pad/accumulation, buffalo hump), (6) Thin skin (thinning of skin, visible subcutaneous blood vessels), (7) Ecchymoses (bruises), (8) Violaceous striae (dark, purple, >1 cm wide, striae), (9) Skin hyperpigmentation (darkening), (10) Buccal (gingival) hyperpigmentation, (11) Peripheral (lower extremity) edema, (12) Hirsutism (terminal, dark hairs, excessive hair growth, beard, male-pattern hair distribution), (13) Acne/folliculitis (erythematous papular rash on face, scalp, or groin), (14) Proximal muscle weakness (inability to rise from seated position or squatting without use of arms,<5/5 strength in deltoids on neurological exam), (15) acanthosis nigricans.
The symptoms and associated conditions of hypercortisolism were quantified from the history of present illness and past medical history sections of the clinic note. For each symptom or condition, the following descriptors were considered to be synonymous and indicative of a positive finding: (1) headache, (2) depression (low mood, crying, tearfulness, sadness), (3) decreased concentration (inability to focus), (4) impaired memory (forgetfulness), (5) insomnia, (6) anxiety/irritability (stressed), (7) fatigue/decreased strength (low energy, difficulty walking up flight of steps or getting up from seated position, muscle weakness, tired), (8) weight gain (inability to lose weight), (9), back pain, (10) facial flushing/fullness (facial redness, weight gain in face, rounding of face, facial puffiness), (11) easy bruisability (frequent bruising), (12) skin hyperpigmentation (darkening, tanning, or tanned appearance), (13) hirsutism/frontal balding (excessive hair growth, shaving, plucking, or cosmetic removal of hair, scalp hair loss), (14) acne/folliculitis (pimples, rash or bumps around hair follicles, itchy rash on scalp, neck, or groin) (15) decreased libido (low sex drive, loss of sexual interest), (16) menstrual irregularities (loss or absence of periods, infrequent or irregular menstrual bleeding), (17) hypertension (established diagnosis), (18) Glucose intolerance/type 2 diabetes (established diagnosis of either impaired fasting glucose, impaired glucose tolerance, or type 2 diabetes mellitus), (19) polycystic ovarian syndrome (established diagnosis), (20) hypokalemia (documented serum potassium less than 3.5 mEq/l or on potassium supplementation), (21) kidney stones (established diagnosis of nephrolithiasis), (22) low bone mineral density (osteopenia, osteoporosis, prior fracture).
The laboratory evaluation of hypercortisolism included tests performed by the two evaluating endocrinologists and those obtained by referring physicians prior to the visit. Over the last 10 years, commercial laboratory assay methodologies have been subject to change. At our institution, serum cortisol has been measured using the same immunoassay for the past 10 years, with the only modification being a change in incubation time, which occurred 5 years ago. Various commercial assays were used in this study for urine free cortisol (UFC) and salivary cortisol measurements. The most commonly used commercial UFC assay utilized high-performance liquid chromatography from 2000 to 2005, and since 2005 has utilized liquid chromatography/mass spectrometry. To compare inter-laboratory results of urine and salivary cortisol measurements, we normalized the values by dividing by the upper limit of the reference range provided by the performing laboratory.
The normalized UFC reflected 11 single values and the average of 3 double values for the MAs patients, and 33 single values and the average of 14 double values for the μAs. The mean normalized salivary cortisol reflected 3 single values and was the average of 7 double values for the MAs; it reflected 3 single values, the average of 16 double values and 1 triple values for the μAs.
Various dexamethasone (Dex) suppression tests (DST) were employed. The overnight 1 mg DST consisted of a serum cortisol measurement obtained the morning after the administration of 1 mg dose of Dex in late evening. The 2-day low dose Dex suppression test (LDDST) consisted of 0.5 mg of Dex administered every 6 h for 48 h, with measurement of either serum cortisol or UFC before and after Dex. The overnight DST was used in 9 MA patients (56%) and 24 μA patients (65%). The 2-day LDDST was used in only 1 MA patient (6%) and 3 μA patients (8%).
Two high dose Dex suppression tests (HDDST) were used: the overnight 8 mg HDDST and the 2-day HDDST. The 8 mg overnight test was used in 5 MA patients (31%) and 20 μA patients (54%). The 2-day test was used in 0 MA patients and 7 μA patients (19%). Given the small number of patients who underwent 2-day LDDST and 2-day HDDST, data were not reported for these tests.
Statistical analysis
GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, California, USA) was employed to perform statistical analysis. T test was used to compare means between the groups, Chi-square test was used for comparison of proportions, and One-way Analysis of Variance (ANOVA) was used in multiple group comparisons with Bonferroni’s post-hoc test. Data are expressed as means ± SEM.
Results
The demographics and characteristics of the patients are summarized in Table 1. The vast majority of patients were female, both in the μA and MA groups. The mean age of the patients was similar. The mean BMI in both groups identified the patients as obese, with a non-significant trend towards a higher BMI in the MAs. Despite similar prevalence of type 2 diabetes between the groups, the MA patients had a higher likelihood of being on insulin therapy. The prevalence of hypertension and the severity of hypertension (as assessed by the number of anti-hypertensive medications required per patient) were similar in the two groups; however we were unable to assess how anti-hypertensive dose or relative potencies of individual anti-hypertensive medications reflected the degree of hypertension. Tumoral compressive symptoms were absent in the μA group, whereas optic chiasm compression was observed in half, and cavernous sinus invasion in one-fourth of the MA patients. There was no significant difference in the likelihood of identifying an adenoma at pathology or surgical cure rate between the two groups.
Table 1.
Patients demographics
| All adenomas n = 53 | μAs n = 37 | MAs n = 16 | P value (μAs vs. MAs) | |
|---|---|---|---|---|
| # female/male (%) | 49/4 (92/8) | 35/2 (95/5) | 14/2 (88/12) | 0.369 |
| Mean age (years) | 38.5 ± 1.8 | 38.1 ± 2.1 | 39.6 ± 3.7 | 0.178 |
| Mean BMI (kg/m2) | 35.6 ± 1.3 (n = 47) | 34.1 ± 1.4 (n = 31) | 40.0 ± 1.8 (n = 16) | 0.127 |
| Mean SBP (mmHg) | 140.4 ± 1.9 (n = 52) | 140.1 ± 2.1 (n = 36) | 141.1 ± 4.1 (n = 16) | 0.800 |
| Mean DBP (mmHg) | 84.0 ± 1.6 (n = 52) | 83.4 ± 1.8 (n = 36) | 85.1 ± 3.1 (n = 16) | 0.623 |
| # with diagnosis of hypertension (%) | 38 (72) | 25 (68) | 13 (81) | 0.310 |
| Mean # anti-hypertensive drug per patient | 1.0 ± 0.2 | 0.9 ± 0.2 | 1.3 ± 0.4 | 0.437 |
| # with diagnosis of type 2 diabetes (%) | 17 (32) | 12 (32) | 5 (31) | 0.933 |
| Mean # oral hypoglycemic per patient | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.3 ± 0.2 | 0.610 |
| % insulin-treated | 9 | 3 | 25 | 0.025 |
| Mean tumor diameter (cm) | 0.79 ± 0.96 | 0.44 ± 0.04 | 1.60 ± 0.18 | <0.0001 |
| % with optic chiasm compression | 15 | 0 | 50 | <0.0001 |
| % with cavernous sinus invasion | 8 | 0 | 25 | <0.01 |
| % with cranial nerve deficit | 2 | 0 | 7 | 0.125 |
| % cured following first surgerya | 69 (n = 49) | 64 (n = 33) | 81 (n = 16) | 0.210 |
| % with adenoma identified at pathology | 84 (n = 49) | 82 (n = 33) | 88 (n = 16) | 0.614 |
| % adenomas with positive ACTH immuno-staining | 83 (n = 47) | 87 (n = 31) | 75 (n = 16) | 0.296 |
BMI body mass index, SDP systolic blood pressure, DBP diastolic blood pressure
Statistically significant P values are in bold
Pathology and outcome unavailable for 2 cases where surgery was pursued at outside institutions, and for 2 cases in which surgery has not yet occurred
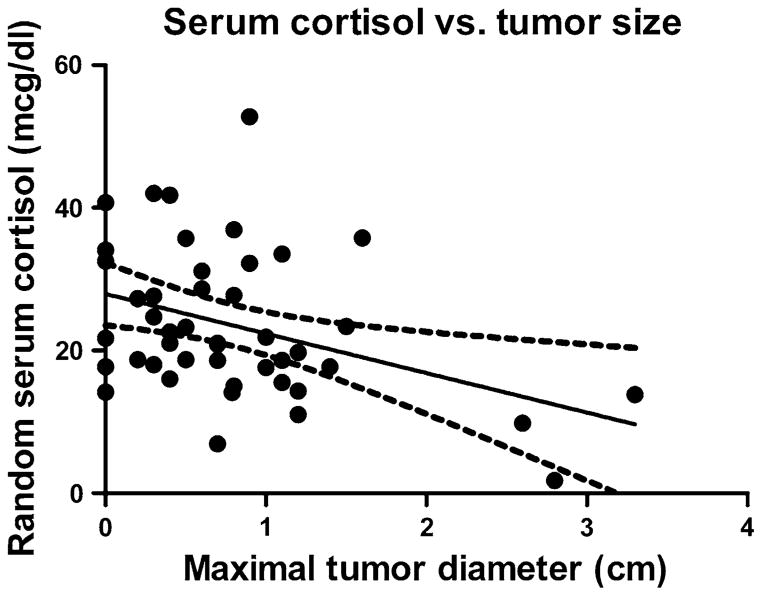
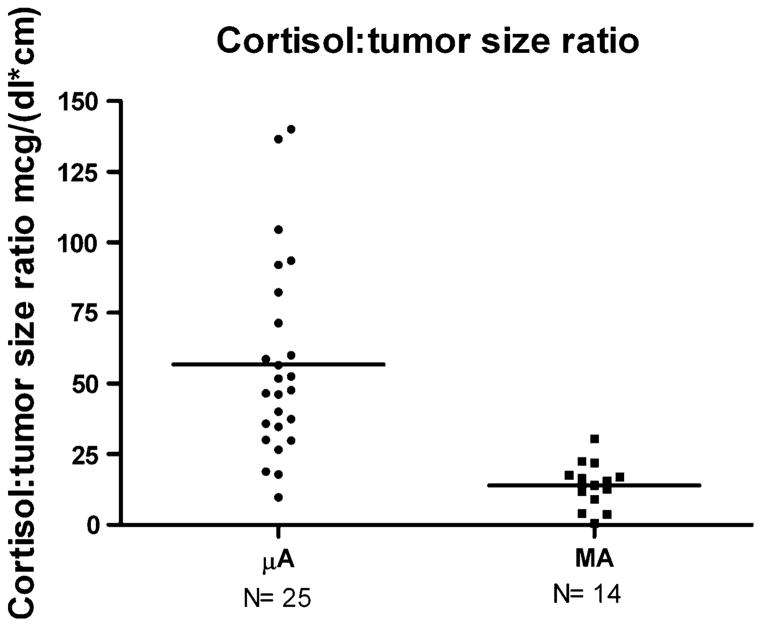
The baseline biochemical parameters are shown in Table 2. A significantly lower random serum cortisol was observed in the MA group, while the random serum ACTH level, 24 h urine free cortisol (UFC), salivary cortisol, mean serum cortisol following 1 mg dexamethasone-suppression test (DST), and percentage suppressibility following 8 mg dexamethasone were not different between the groups. Cortisol levels decreased as a function of tumor size (r2 = 0.15, P = 0.0097) (Fig. 1), and the cortisol:tumor diameter ratio was significantly lower in the MA group (14.1 ± 2.2 vs. 56.8 ± 7.2 mcg/(dl × cm), P <0.01) (Fig. 2).
Table 2.
Biochemical parameters
| All adenomas n = 53 | μAs n = 37 | MAs n = 16 | P value μA vs. MA | |
|---|---|---|---|---|
| Random ACTH (pg/ml) | 71.9 ± 4.6 (n = 50) | 69.5 ± 5.3 (n = 36) | 78.3 ± 8.9 (n = 14) | 0.390 |
| Random cortisol (mcg/dl) | 23.5 ± 1.5 (n = 45) | 25.9 ± 1.8 (n = 31) | 18.2 ± 2.4 (n = 14) | 0.018 |
| Cortisol:ACTH ratioa | 0.37 ± 0.03 (n = 48) | 0.42 ± 0.05 (n = 34) | 0.25 ± 0.03 (n = 14) | 0.048 |
| Mean fold elevation in 24 h UFC | 4.4 ± 0.9 (n = 44) | 4.9 ± 1.1 (n = 33) | 2.9 ± 0.9 (n = 11) | 0.34 |
| Mean fold elevation in salivary cortisol | 4.6 ± 0.8 (n = 26) | 4.9 ± 1.1 (n = 20) | 3.1 ± 0.7 (n = 6) | 0.36 |
| Mean serum cortisol after 1 mg DST (mcg/dl) | 15.7 ± 1.6 (n = 30) | 16.7 ± 1.9 (n = 20) | 14.1 ± 2.7 (n = 10) | 0.49 |
| Mean serum cortisol after 8 mg DST (mcg/dl) | 6.29 ± 1.37 (n = 25) | 7.41 ± 1.62 (n = 20) | 1.83 ± 0.58 (n = 5) | 0.10 |
| % suppressibility in serum cortisol after 8 mg DST | 74.3 ± 5.4 (n = 25) | 71.5 ± 5.9 (n = 20) | 90.2 ± 2.3 (n = 5) | 0.14 |
Statistically significant P values are in bold
Values are means ± SEM
Derived from simultaneous measurements of random serum ACTH and cortisol
Fig. 1.
Serum cortisol as a function of maximal tumor diameter. r2 = 0.15, P = 0.0097
Fig. 2.
Serum cortisol: tumor diameter ratio. P < 0.0001
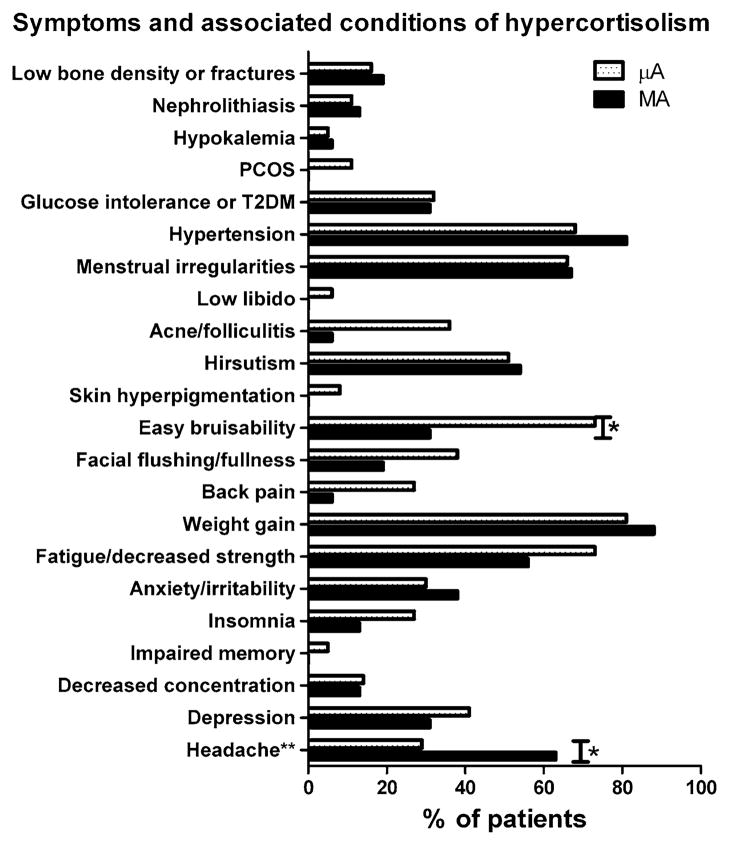
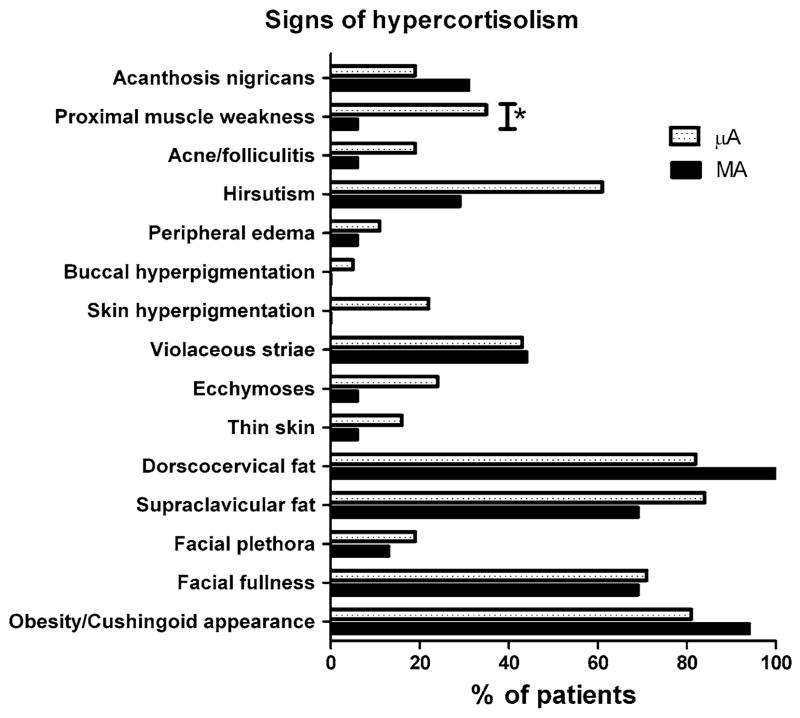
The prevalence of signs, symptoms/associated conditions, and clinical manifestations of hypercortisolism is shown in Table 3. The prevalence of individual symptoms and signs is shown in Figs. 3 and 4. There was a higher prevalence of easy bruisability in the μA group, and higher prevalence of headache in the MA group (Fig. 3). A higher prevalence of proximal muscle weakness was seen in the μA group (Fig. 4).
Table 3.
Prevalence of clinical findings by study group. Values are means ± SEM
| All adenomas n = 53 | μAs n = 37 | MAs n = 16 | P value μA vs. MA | |
|---|---|---|---|---|
| Mean # signs (% of total assessed) | 5.6 ± 1.7 (37.2 ± 1.6) | 5.9 ± 0.3 (39.4 ± 1.9) | 4.8 ± 0.3 (31.9 ± 2.1) | 0.026 |
| Mean # symptoms (% of total assessed) | 7.1 ± 0.4 (32.0 ± 1.6) | 7.6 ± 0.4 (33.8 ± 1.9) | 6.0 ± 0.7 (27.7 ± 2.8) | 0.046 |
| Mean # of signs and symptoms (% of total assessed) | 12.6 ± 0.5 (34.1 ± 1.3) | 13.5 ± 0.6 (36.1 ± 1.5) | 10.8 ± 0.9 (29.4 ± 2.2) | 0.019 |
Fig. 3.
Prevalence of symptoms and associated conditions of hypercortisolism. *P < 0.05 between groups. ** = mass effect symptoms
Fig. 4.
Prevalence of signs of hypercortisolism. *P < 0.05 between groups
Discussion
It is commonly assumed that larger secreting pituitary tumors cause a more marked hypersecretory phenotype; however, contrary to the size-dependence observed with other hormone-secreting pituitary adenomas, we did not find a direct relationship between tumor size and clinical phenotype in our cohort of ACTH-secreting adenomas. Indeed, in MA patients, the clinical manifestations of Cushing’s syndrome were not more pronounced than in μAs. Although the MA patients had a greater likelihood of insulin-dependence, these patients in fact had slightly fewer overall signs and clinical manifestations compared to the μAs.
While the prevalence of signs of hypercortisolism was generally similar between the two groups, proximal muscle weakness was observed more commonly in the μA patients. Proximal muscle weakness is considered one of the more discriminating signs of hypercortisolism [13]; this may suggest that the μA patients have a more severe degree of hypercortisolemia. The prevalence of self-reported symptoms and associated conditions of cortisol excess was generally quite similar between groups, with the exception easy bruisability (higher in μAs) and headache (higher in MAs). The higher prevalence of headache in MAs likely reflects increased tumoral mass effect, and is not considered a manifestation of hypercortisolism per se; however, easy bruisability, a highly specific feature of Cushing’s syndrome [13], was reported twice as often by the μA patients compared to the MAs. Despite this, ecchymoses and skin thinning were not observed more frequently on physical examination in these patients. It is worth noting that the number of clinical manifestations of hypercortisolism in our μA patients is lower than what has been reported by Raverot et al. in their cohort of 36 ACTH-secreting adenomas [12]: they found an average of 12 clinical manifestations in the MA patients compared with 21 in the μAs, while we found an average of approximately 11 and 13 clinical manifestations in the MAs and μAs, respectively.
The higher insulin dependence seen in the MA group may reflect a greater degree of insulin resistance in this group, which had a tendency towards a higher BMI. While it seems reasonable to infer a longer duration of hypercortisolemia in the MA patients as the cause of these metabolic derangements, the mean age of the MAs was not different than the μAs. In this regard, our study differs from other groups who have found that μA patients are on average 8–11 years younger than their MA counterparts [4, 11]. In light of the similar 24 h UFC and salivary cortisol values between the two groups and the frankly lower mean serum cortisol level in the MA group, it seems unlikely that the higher insulin resistance is due to a more severe degree of hypercortisolemia. The significance of this finding requires further study.
A linear relationship between baseline ACTH levels and tumor size has been shown in several studies [4, 11, 14, 15]. However, we found no correlation between these variables in our series. Instead, we found that μA patients had higher random serum cortisol levels and higher serum cortisol:ACTH ratios than MAs, and, despite being smaller, their UFC and salivary cortisol values were not lower than in MAs. It is important to emphasize that the serum hormonal measurements obtained in our study were random, and it is certainly possible that the lack of standardized timed blood draws may have masked a relationship. Indeed, Woo et al. identified both higher morning plasma ACTH and cortisol levels in a retrospective study of ACTH-MAs [4]. We observed an inverse relationship between cortisol and tumor size, suggesting reduced cortisol production in patients with larger tumors. Additionally, contrary to prior reports showing reduced suppressibility during high dose DST in MAs, we did not find any variability in responsiveness to exogenous glucocorticoids with respect to tumor size [6, 11].
Since several studies have demonstrated that ACTH-MAs may be more indolent, it has been speculated that the ACTH produced by MAs may be immunoreactive but less biologically active than that produced by μAs [4]. Our data seems to support this theory. Interestingly, the mean cortisol:ACTH ratio we observed in the MA group is in keeping with the findings of Raverot et al., who have shown that silent corticotroph adenomas (SCA) and ACTH-MAs have similar ratios of cortisol:ACTH production [12]. Their study found a high degree of similarity between SCAs and MAs: (1) older age at diagnosis, (2) higher percentage of invasive tumors and rate of recurrence, (3) lower cortisol:ACTH ratio compared to μAs, (4) variability of cytological differentiation and immunoreactivity of the three peptides of POMC, (5) molecular variability of TPIT and POMC gene expression, and (6) low galectin-3 expression at the mRNA level [12]. Thus, larger ACTH-secreting adenomas may be less able to secrete ACTH, behaving as “quiet” rather than “silent” tumors. Another plausible explanation for differences in clinical phenotype might be variations in ACTH pulsatility between μAs and MAs or indirect effects at the level of the adrenal gland. To explore these differences, it would be interesting to examine the biological activity of ACTH derived from the plasma of CD patients on adrenocortical cells in vitro.
Interestingly, we found a higher proportion of MAs (30%) than expected based on previous prevalence estimates (4–23.3%) of MAs as the cause of CD [4, 5, 16]. This may reflect different testing strategies in different health care environments, but may also indicate that closer attention to milder cases of CD may be required so as not to mistake these tumors for non-functioning adenomas. The possible merits of obtaining baseline ACTH levels as a screening test in patients with pituitary incidentalomas to detect cases of SCAs (plasma ACTH levels may be elevated in patients harboring these tumors despite the lack of clinical manifestations of cortisol excess) is discussed briefly in the recent Endocrine Society guideline on the evaluation of pituitary incidentalomas [15]. Similarly, a low threshold for hypercortisolemia testing may be appropriate in cases of pituitary MAs even when only mild signs or symptoms of hypercortisolism, such as obesity alone, are present.
Finally, it has been reported by Blevins et al. that the remission rate is significantly lower in MAs than in μAs patients [16]. We have found no difference in remission rate. One possible explanation is that in the Blevins’s study all MA patients had typical clinical features of CD, and therefore their outcome may reflect more what happens to MAs that are more efficient in secreting biologically active ACTH.
Limitations of our study include disproportionate numbers within the groups (μAs vs. MAs), the retrospective nature, and the lack of standardized timing of hormonal measurements. Furthermore, observational bias may be present: since the evaluation for hypercortisolism was triggered by a clinical suspicion, we may have missed even milder cases. This would, however, only strengthen our conclusions. We recognize that another major limitation of a retrospective study of this kind is the lack of standardization in quantification of signs/symptoms. However, the fact that the majority of cases (both μA and MA) were seen by a single provider should have yielded uniformity of judgment, compensating in some measure for the inherent flaws of a retrospective study.
Our study illustrates the fact that ACTH-secreting adenomas may occur along a spectrum ranging from “silent” to “quiet” to overt secretors. Consequently, the diagnosis of CD should not be dismissed on the basis of a paucity of clinical manifestations in the setting of a MA. Given the potential for post-operative adrenal insufficiency in patients with ACTH-secreting MAs who undergo surgical resection, recognizing this subset of CD patients is vital.
Acknowledgments
N.M. was supported by NIH T32 DK007751. C.P. was supported by a Howard Hughes Medical Student research fellowship, A.Q. was supported in part by NIH grant K08 NS 055851 and Howard Hughes Medical Institute grant 905675.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Nestoras Mathioudakis, Division of Endocrinology, Department of Medicine, The Johns Hopkins University, Baltimore, MD 21287, USA.
Courtney Pendleton, Department of Neurosurgery, The Johns Hopkins University, Baltimore, MD 21287, USA.
Alfredo Quinones-Hinojosa, Department of Neurosurgery, The Johns Hopkins University, Baltimore, MD 21287, USA.
Gary S. Wand, Division of Endocrinology, Department of Medicine, The Johns Hopkins University, Baltimore, MD 21287, USA
Roberto Salvatori, Email: salvator@jhmi.edu, Division of Endocrinology, Department of Medicine, The Johns Hopkins University, Baltimore, MD 21287, USA. Division of Endocrinology and Metabolism, Johns Hopkins University School of Medicine, 1830 E. Monument St #333, Baltimore, MD 21287, USA.
References
- 1.Bertagna X, Guignat L, Groussin L, Bertherat J. Cushing’s disease. Best Pract Res Clin Endocrinol Metab. 2009;23:607–623. doi: 10.1016/j.beem.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Steffensen C, Bak AM, Rubeck KZ, Jorgensen JO. Epidemiology of Cushing’s syndrome. Neuroendocrinology. 2010;92(Suppl 1):1–5. doi: 10.1159/000314297. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann BM, Hlavac M, Martinez R, Buchfelder M, Muller OA, Fahlbusch R. Long-term results after microsurgery for Cushing disease: experience with 426 primary operations over 35 years. J Neurosurg. 2008;108:9–18. doi: 10.3171/JNS/2008/108/01/0009. [DOI] [PubMed] [Google Scholar]
- 4.Woo YS, Isidori AM, Wat WZ, Kaltsas GA, Afshar F, Sabin I, Jenkins PJ, Monson JP, Besser GM, Grossman AB. Clinical and biochemical characteristics of adrenocorticotropin-secreting macroadenomas. J Clin Endocrinol Metab. 2005;90:4963–4969. doi: 10.1210/jc.2005-0070. [DOI] [PubMed] [Google Scholar]
- 5.Hwang YC, Chung JH, Min YK, Lee MS, Lee MK, Kim KW. Comparisons between macroadenomas and microadenomas in Cushing’s disease: characteristics of hormone secretion and clinical outcomes. J Korean Med Sci. 2009;24:46–51. doi: 10.3346/jkms.2009.24.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannavo’ S, Almoto B, Dall’Asta C, Corsello S, Lovicu RM, De Menis E, Trimarchi F, Ambrosi B. Long-term results of treatment in patients with ACTH-secreting pituitary macroadenomas. Eur J Endocrinol. 2003;149:195–200. doi: 10.1530/eje.0.1490195. [DOI] [PubMed] [Google Scholar]
- 7.Storr HL, Alexandraki KI, Martin L, Isidori M, Kaltsas GA, Monson JP, Besser GM, Matson M, Evanson J, Afshar F, Sabin I, Savage MO, Grossman AB. Comparisons in the epidemiology, diagnostic features and cure rate by transsphenoidal surgery between paediatric and adult-onset Cushing’s disease. Eur J Endocrinol. 2011;164:667–674. doi: 10.1530/EJE-10-1120. [DOI] [PubMed] [Google Scholar]
- 8.Krzentowska-Korek A, Golkowski F, Baldys-Waligorska A, Hubalewska-Dydejczyk A. Efficacy and complications of neurosurgical treatment of acromegaly. Pituitary. 2011;14:157–162. doi: 10.1007/s11102-010-0273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babey M, Sahli R, Vajtai I, Andres RH, Seiler RW. Pituitary surgery for small prolactinomas as an alternative to treatment with dopamine agonists. Pituitary. 2011;14:222–230. doi: 10.1007/s11102-010-0283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart PM, Petersenn S. Rationale for treatment and therapeutic options in Cushing’s disease. Best Pract Res Clin Endocrinol Metab. 2009;23(Suppl 1):S15–22. doi: 10.1016/S1521-690X(09)70004-1. [DOI] [PubMed] [Google Scholar]
- 11.Selvais P, Donckier J, Buysschaert M, Maiter D. Cushing’s disease: a comparison of pituitary corticotroph microadenomas and macroadenomas. Eur J Endocrinol. 1998;138:153–159. doi: 10.1530/eje.0.1380153. [DOI] [PubMed] [Google Scholar]
- 12.Raverot G, Wierinckx A, Jouanneau E, Auger C, Borson-Chazot F, Lachuer J, Pugeat M, Trouillas J. Clinical, hormonal and molecular characterization of pituitary ACTH adenomas without (silent corticotroph adenomas) and with Cushing’s disease. Eur J Endocrinol. 2010;163:35–43. doi: 10.1530/EJE-10-0076. [DOI] [PubMed] [Google Scholar]
- 13.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda H, Yoshimoto T, Ogawa Y, Mizoi K, Murakami O. Clinico-pathological study of Cushing’s disease with large pituitary adenoma. Clin Endocrinol (Oxf) 1997;46:669–679. doi: 10.1046/j.1365-2265.1997.1741013.x. [DOI] [PubMed] [Google Scholar]
- 15.Freda PU, Beckers AM, Katznelson L, Molitch ME, Montori VM, Post KD, Vance ML. Pituitary incidentaloma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:894–904. doi: 10.1210/jc.2010-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blevins LS, Jr, Christy JH, Khajavi M, Tindall GT. Outcomes of therapy for Cushing’s disease due to adrenocorticotropin-secreting pituitary macroadenomas. J Clin Endocrinol Metab. 1998;83:63–67. doi: 10.1210/jcem.83.1.4525. [DOI] [PubMed] [Google Scholar]