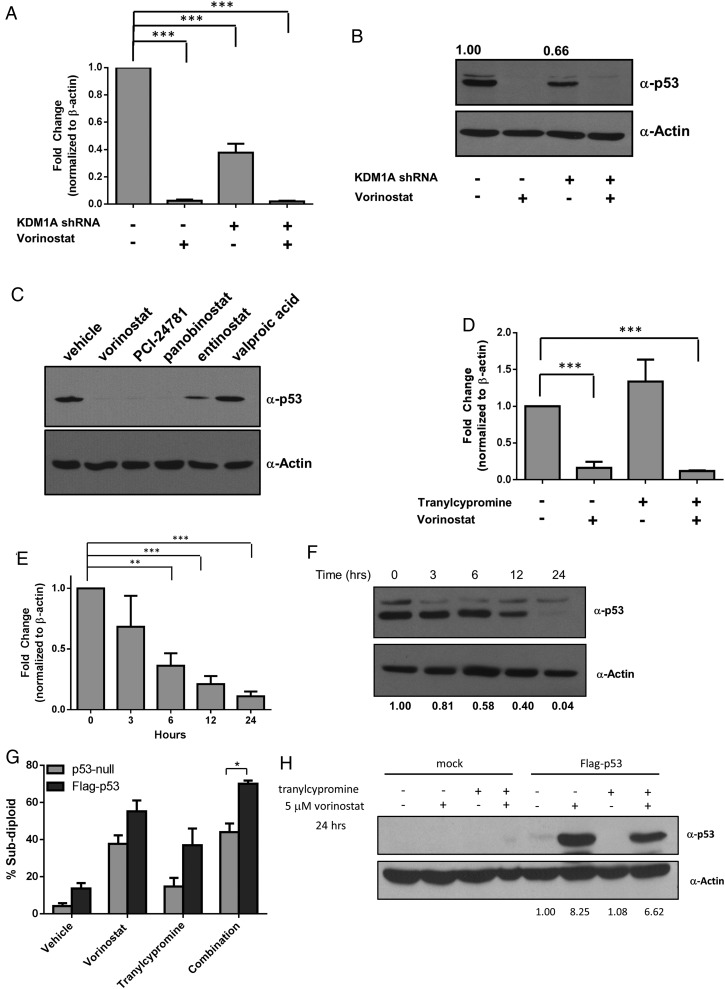
Fig. 3.
p53 mRNA and protein expression is rapidly regulated by HDAC and KDM1A inhibition. LN-18 cells transfected with control or KDM1A shRNA were treated with 5 µM vorinostat for 24 hours, and (A) TP53 gene expression or (B) p53 protein expression was measured. (C) LN-18 cells were treated with 5 µM of the HDACi indicated. p53 protein expression was evaluated 24 hours after treatment. (D) LN-18 cells were treated with 5 µM vorinostat, 1 mM tranylcypromine, or the combination for 24 hours, and p53 mRNA expression was evaluated. (E) p53 mRNA was measured in LN-18 cells treated with 5 µM vorinostat at the time points indicated. (F) p53 protein was assessed after treatment with 5 µM vorinostat by Western blot at the indicated time points. (G) LNZ308 (p53-null) cells were transfected with empty vector or vector-expressing wild-type p53 (Flag-p53). DNA fragmentation was measured 72 hours after treatment with 5 µM vorinostat, 1 mM tranylcypromine, or the combination. (H) Western blots demonstrating lack of p53 protein in LNZ308 cells and ectopic expression of wild-type p53 protein under conditions stated in part G. All Western blots are representative of 3 independent experiments. Actin was used as a loading control. n = 3, mean ± SEM. *P ≤ .05, ** P ≤ .01, ***P ≤ .001.