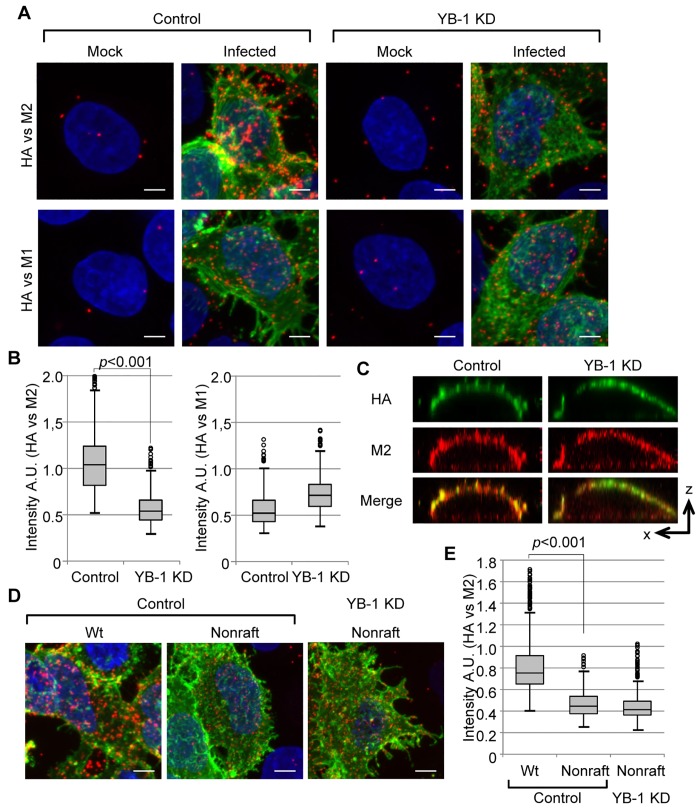
Fig 7. Clustering of HA and M2 requires ERC formation mediated by YB-1.
(A, B, and C) in situ PLA assays using wild-type virus. At 48 h post transfection with either non-targeting or YB-1 siRNA, HeLa cells were infected with wild-type influenza virus at MOI of 10. At 8 h post infection, cells were fixed and subjected to in situ PLA assays with anti-HA and either anti-M1 or anti-M2 antibodies without permeabilization in 0.5% Triton X-100 (red). HA and DNA were counter-stained with anti-mouse IgG conjugated with Alexa 488 (green) and DAPI (blue), respectively. In panel B, the mean intensity of each punctate PLA signal obtained from three independent experiments was quantitated using IMARIS software (n>5,000). In panel C, cells were subjected to the indirect immunofluorescence assays with anti-HA (green) and anti-M2 antibodies (red). The single optical sections in the x-z plane are taken. (D and E) in situ PLA assays using nonraft HA virus. Control and YB-1 KD cells were infected with a mutant virus at MOI of 10, which has alanine substitutions at I533, Y534, and S535 in the transmembrane domain of HA (nonraft), and subjected to in situ PLA assays with anti-HA and anti-M2 antibodies (red). HA and DNA were counter-stained with anti-mouse IgG conjugated with Alexa 488 (green) and DAPI (blue), respectively. In panel E, the mean intensity of each punctate PLA signal obtained from three independent experiments was quantitated using IMARIS software (n>5,000). The stacking images along the z-axis were obtained by Maximum intensity projection processing of ZEN 2009 software (Carl Zeiss) (panel A and D). The level of significance was determined by Student’s t test. Scale bar, 5 μm.