Figure 1. MC1R signaling controls repair of UV-induced DNA damage and suppression of mutations via elevation of intracellular levels of cyclic AMP.
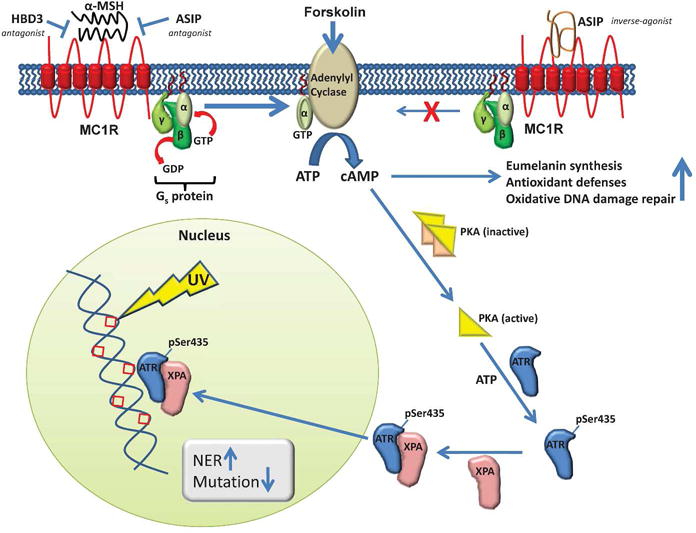
MC1R is a G-protein-coupled 7-pass transmembrane receptor expressed on the cell surface of melanocytes. Upon binding the agonist alpha-melanocyte-stimulating hormone (α-MSH), conformational changes in MC1R elicit the activation of the heterotrimeric G-protein Gs by exchange of bound GDP for GTP. The α-subunit of the G-protein (bound to GTP) can then activate adenylyl cyclase, which in turn catalyzes the cyclization of ATP to give cyclic AMP (cAMP). ASIP and HBD3 interfere with the binding of α-MSH to MC1R (top left). There is also some baseline activity of unstimulated MC1R, which is negatively affected by inverse-agonist activity of ASIP (top right). cAMP interacts with the inactive heterotetrameric form of PKA, freeing the catalytically active monomeric catalyze the phosphorylation of ATR on serine 435 to give ATR(pSer435). The physical interaction between ATR(pSer435) and XPA drives the recruitment of XPA to sites of nuclear photodamage, thereby facilitating repair of the DNA and decreased rates of mutation.
