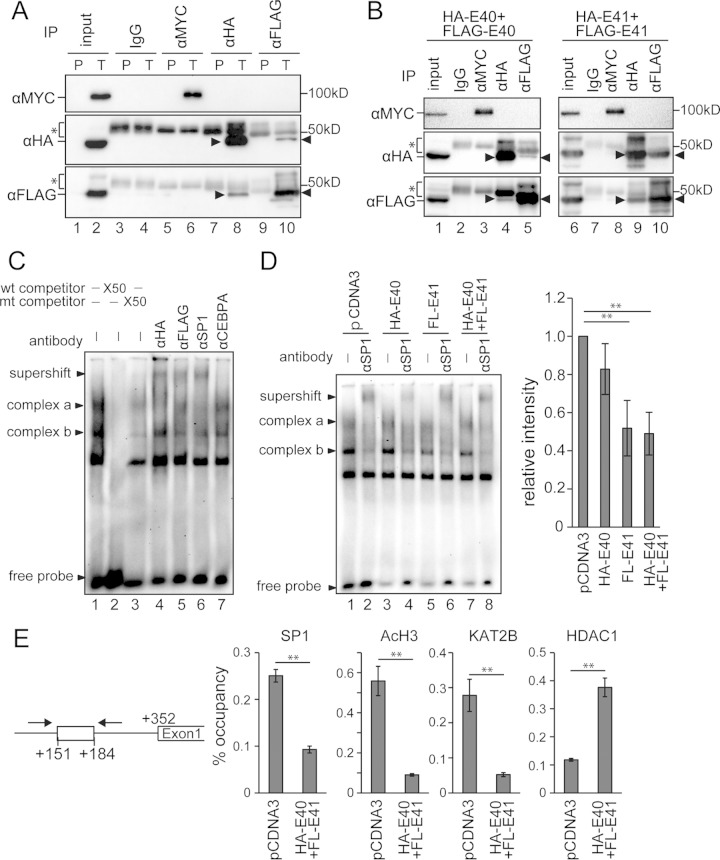
FIG 7.
BHLHE40/41 suppressed SP1 function by a competitive mechanism for binding to the SBS of the TWIST1 promoter. (A) Immunoprecipitation (IP) assay using 293T cells transfected with MYC-SP1, HA-BHLHE40, and FLAG-BHLHE41. The cell lysate was immunoprecipitated with the antibodies shown at the top and immunoblotted with the antibodies indicated on the left (α, anti-). P, cells transfected with pCDNA3 alone; T, cells transfected with all three constructs. (B) IP assay using 293T cells transfected with MYC-SP1, HA-BHLHE40, and FLAG-BHLHE40 (left panels) or 293T cells transfected with MYC-SP1, HA-BHLHE41, and FLAG-BHLHE41 (right panels). The arrowheads indicate the target bands. The bands indicated by asterisks are IgG heavy chains. (C) Electrophoretic mobility shift assay using the nuclear extract of 293T cells transfected with HA-BHLHE40 and FLAG-BHLHE41. Two main SP1-DNA complexes (complexes a and b) were formed. Supershift bands were formed by incubation with anti-HA, -FLAG, and -SP1 antibodies. An anti-CEBPA antibody was used as a negative control. wt, wild type; mt, mutant. (D) Nuclear extracts from 293T cells transfected with HA-BHLHE40 and/or FLAG-BHLHE41 were incubated with the labeled SBS probe. An anti-SP1 antibody was used to form supershift bands. The right graph shows the quantified band intensities of complex b in lanes 1, 3, 5, and 7 from four independent experiments. (E) Chromatin immunoprecipitation assay using 293T cells transfected with HA-BHLHE40 and FLAG-BHLHE41. Protein-DNA complexes immunoprecipitated with each of the anti-SP1, -acetylated H3, -KAT2B, and -HDAC1 antibodies were used to amplify the SBS site by PCR. The 10% input samples were used to calculate the occupancy ratio (%) from the values measured by real-time PCR. **, P < 0.01.