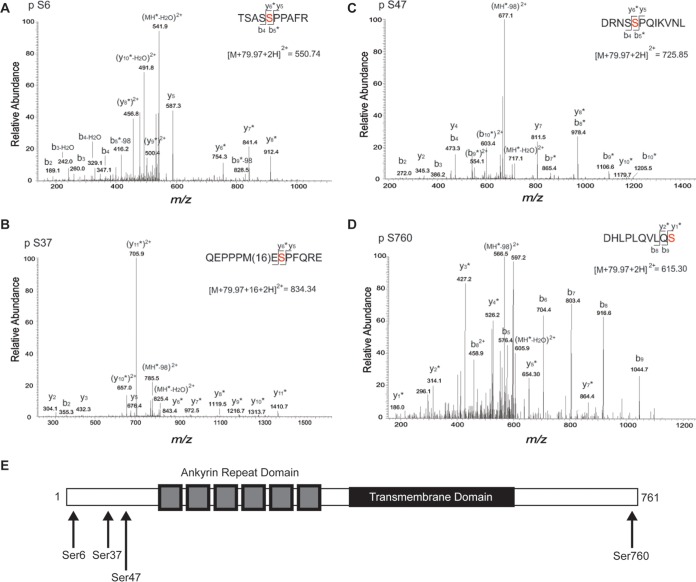
FIG 8.
Mass spectrometry analysis of heterologously expressed TRPV2 to determine phosphorylation sites. (A) MS/MS spectra of the phosphorylated form of the TSASSPPAFR peptide (positions 2 to 11) observed as a doubly protonated ion at m/z 550.74. The presence of y6-y8, b5-98, and b9-98 fragment ions with a 79.97-Da mass shift shows that modification of this peptide occurred at S6. Asterisks indicate the fragment ions that were modified by 79.97 Da. (B) MS/MS spectra of the modified form of the QEPPPMESPFQRE peptide (positions 30 to 42) observed as a doubly protonated ion at m/z 834.34. The presence of y6-y7 fragment ions with a 79.97-Da mass shift and y8-y11 ions with 79.97-Da and 16-Da mass shifts shows that phosphorylation and oxidation of this peptide occurred at positions S37 and M35, respectively. Asterisks indicate the fragment ions that were modified by 79.97 Da. (C) MS/MS spectra of the phosphorylated form of the DRNSSPQIKVNL peptide (positions 43 to 54) observed as a doubly protonated ion at m/z 725.85. The presence of y8-y10 and b5-b10 fragment ions with a 79.97-Da mass shift shows that modification of this peptide occurred at S47. Asterisks indicate the fragment ions that were modified by 79.97 Da. (D) MS/MS spectra of the phosphorylated form of the DHLPLQVLQS peptide (positions 751 to 760) observed as a doubly protonated ion at m/z 615.30. The presence of y1-y7 fragment ions with a 79.97-Da mass shift and unmodified b5-b9 fragment ions shows that modification of this peptide occurred at S760. Asterisks indicate the fragment ions that were modified by 79.97 Da. (E) Domain structure of a TRPV2 monomer. TRPV2 consists of large soluble N and C termini. The N terminus contains six ankyrin repeats that form the ankyrin repeat domain. The four predicted ERK phosphorylation sites (indicated by arrows) reside on the distal N and C termini.